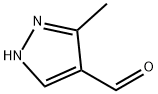
3-METHYL-1H-PYRAZOLE-4-CARBALDEHYDE synthesis
- Product Name:3-METHYL-1H-PYRAZOLE-4-CARBALDEHYDE
- CAS Number:112758-40-4
- Molecular formula:C5H6N2O
- Molecular Weight:110.11
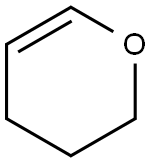
110-87-2
522 suppliers
$10.00/1g
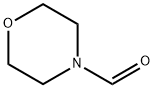
4394-85-8
307 suppliers
$6.00/25g
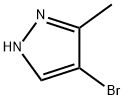
13808-64-5
203 suppliers
$6.00/1g

112758-40-4
151 suppliers
$19.00/250mg
Yield: 8 g
Reaction Conditions:
Stage #1:3,4-dihydro-2H-pyran;4-bromo-3-methyl-1H-pyrazole with trifluoroacetic acid for 12 h;Reflux;
Stage #2: in tetrahydrofuran at -75; for 0.5 h;
Stage #3:4-morpholinecarboxaldehydeFurther stages;
Steps:
2.2
(1) Combine 16.1 g (0.1 mol) of 4-bromo-3-methyl-1H-pyrazole, 10.1 g (0.12 mol) of 3,4-dihydro-2H-pyran, and 0.7 g (0.006 mol) of trifluoroacetic acid ) Add to the reaction bottle and stir to heat to micro-reflux for 12 hours. The reaction formula is as follows:Cool to room temperature, add 0.24 g (0.006 mol) of sodium hydride to neutralize the reaction system,Vacuum distillation to obtain intermediate4-bromo-3-methyl-1- (tetrahydro-2H-pyran-2-yl) -1H-pyrazole and4-bromo-5-methyl-1- (tetrahydro-2H-pyran-2-yl) -1H-pyrazole total 24 grams,The presence of isomers does not affect the next reaction. (2) The intermediates obtained in step (1) 4-bromo-3-methyl-1- (tetrahydro-2H-pyran-2-yl) -1H-pyrazole and 4-bromo-5- Methyl-1- (tetrahydro-2H-pyran-2-yl) -1H-pyrazole 24 g was dissolved in 200 ml of THF, cooled to minus 75 degrees, and n-butyl with a concentration of 2.5 mol / L was added dropwise 48 ml (0.12 mol) of lithium solution, maintaining the temperature at minus 75 degrees Celsius, dropping and holding for half an hour, then adding 14 g (0.12 mol) of N-formylmorpholine at this temperature, the reaction formula in step (2) is as follows :After the reaction is completed, naturally warm to room temperature, add dilute sulfuric acid to quench until the system pH value is less than 1, stir at room temperature for 3-4 hours, neutralize with sodium bicarbonate, extract with ethyl acetate, dry and concentrate to obtain the target product by adsorption and purification on silica gel 3-Methyl-1H-pyrazole-4-carbaldehyde 8 g.The yield of the target product 3-methyl-1H-pyrazole-4-carbaldehyde in this example was 72.7%. 2 is the nuclear magnetic resonance spectrum of the product 3-methyl-1H-pyrazole-4-carbaldehyde obtained in this example;
References:
Changsha Luxing Biological Technology Co., Ltd.;Tan Yongjun;Yang Bing;Zhu Zhiping;Zhang Jianhua CN110734401, 2020, A Location in patent:Paragraph 0030-0039
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

112758-40-4
151 suppliers
$19.00/250mg

112758-40-4
151 suppliers
$19.00/250mg
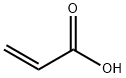
79-10-7
700 suppliers
$20.16/100 mL