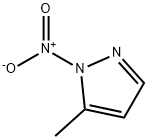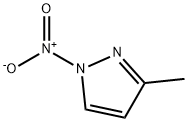
3-methyl-1-nitro-1h-pyrazole synthesis
- Product Name:3-methyl-1-nitro-1h-pyrazole
- CAS Number:31163-84-5
- Molecular formula:C4H5N3O2
- Molecular Weight:127.1
Yield:-
Reaction Conditions:
Stage #1: 3-Methylpyrazolewith nitro acetate;acetic acid at -5 - 5;
Stage #2: with sodium carbonate in water; pH=~ 7;
Steps:
1
Synthesis of the intermediate 3-methyl-5-nitro-1H-pyrazole 9; [00136] Acetic anhydride (96.2 mL, 104.58 g, 1.02 mol) was cooled to -15° C with an ice / NaCl bath and to this was dropwise, very slowly, added red fuming HNO3 (40.7 mL, 61.8 g, 0.98 mmol). The reaction is strongly exothermic and care must be taken to keep the temperature below 0°C (inside temperature). This solution was then rapidly transferred with a teflon tube of sufficient diameter (to prevent warming on transfer) to an addition funnel with a cooling mantle. This solution was kept <;0° C at all times and the addition funnel mounted on a flask containing methyl pyrazole (35 g, 0.427 mol) in glacial acetic acid (40 mL) at -5° C. The chilled (-15° C) acetyl nitrate solution was then dropwise added to the methyl pyrazole 8 solution at 0° C. The reaction was highly exothermic until ca. 1 eq. acetyl nitrate had been added and it must not be allowed to warm above 5° C. After 3 hours stirring at 0° C the mixture was poured into ice / water (500 mL) and neutralized to ca. pH 7 with sodium carbonate. Extraction with DCM (3x 200 mL), drying (sodium sulfate) and evaporation in vacuo afforded a 90:10 mixture of N-nitro intermediates (42.8 g, 0.337 mol, 79%), which was dissolved in perchloroethylene (4 L), heated at 140 °C ext. temperature for 18 hours and for 5 hours at 165° C. Evaporation of the solvent and drying in vacuo gave pure 9 (39.0 g, 0.307 mol, 72% both steps). [00137] It should be noted that nitration of methyl pyrazoles with HNO3 normally does not proceed at the 3 position and often dinitration is found (S. A. Shevelev, I. L. Dalinger: Russ. J. Org. Chem. 34 (1998) 1071-80). Therefore acetyl nitrate had to be used to affect first the N-nitro compounds (J. W. A. M. Janssen et al.,: J. Org. Chem 38 (1973) 1777-82). These N-nitro compounds were then rearranged by heating to the 3-nitro compounds via an anionotropic 1,5-shift ((a) see Shevelev and Dalinger (1998), (b) J. W. A. M. Janssen, C. L. Habraken: J. Org. Chem. 36 (1971) 3081-4). Mixtures of the two possible N-nitro compounds were observed in the nitration, however the 1 ,5-rearrangement led to interconversion, thus only one product (3 methyl-5-nitro-1H-pyrazole 9) was finally observed.
References:
WO2010/96115,2010,A1 Location in patent:Page/Page column 53-54
1453-58-3
371 suppliers
$6.00/5g

31163-84-5
6 suppliers
inquiry