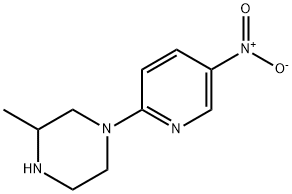
3-METHYL-1-(5-NITROPYRIDIN-2-YL)PIPERAZINE synthesis
- Product Name:3-METHYL-1-(5-NITROPYRIDIN-2-YL)PIPERAZINE
- CAS Number:773879-30-4
- Molecular formula:C10H14N4O2
- Molecular Weight:222.24
Yield:773879-30-4 89%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 0 - 20;
Steps:
13.1
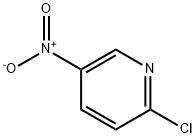
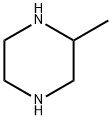
Example 13 N-[6-(4-Allyl-3-methylpiperazin-1-yl)pyridin-3-yl]-4-isopropylbenzenesulfonamide hydrochloride 13.1 3-Methyl-1-(5-nitropyridin-2-yl)piperazine 872 mg (6.31 mmol) of potassium carbonate were added to a solution of 500 mg (3.15 mmol) of 2-chloro-5-nitropyridine in 7 ml of dimethylformamide. After that, a solution of 350 mg (3.32 mmol) of 2-methylpiperazine in 3 ml of dimethylformamide was slowly added dropwise to the reaction mixture while cooling with ice (exothermic reaction). The reaction mixture was stirred for 1 hour while cooling with ice and then stirred overnight at room temperature. After the solvent had been evaporated to dryness, the residue was taken up in water and this mixture was extracted three times with diethyl ether. The combined organic phases were dried over sodium sulfate, filtered and evaporated to dryness, with 3-methyl-1-(5-nitropyridin-2-yl)piperazine (Yield: 650 mg, 89% of theory) being obtained. 1H-NMR (500 MHz, CDCl3): δ [ppm] 9.0 (s, 1H); 8.2 (d, 1H); 6.6 (d, 1H), 4.4 (m, 2H); 3.2 (m, 1H); 3.1 (m, 1H); 2.9 (m, 2H); 2.7 (m, 1H); 1.2 (m, 3H). 13C-NMR (125 MHz, CDCl3): 160.4 (C); 146.5 (CH); 134.9 (C); 133.0 (C); 104.5 (CH); 52.2 (CH2); 50.6 (CH); 45.7 (CH2); 45.4 (CH2); 19.6 (CH3).
References:
US2006/160809,2006,A1 Location in patent:Page/Page column 27