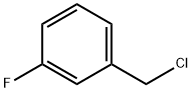
3-Fluorobenzyl chloride synthesis
- Product Name:3-Fluorobenzyl chloride
- CAS Number:456-42-8
- Molecular formula:C7H6ClF
- Molecular Weight:144.57
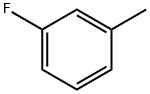
352-70-5
257 suppliers
$12.00/25mL

456-42-8
332 suppliers
$5.00/5g
Yield:456-42-8 35%
Reaction Conditions:
with methanol;tetrachloromethane;iron(II) chloride tetrahydrate;formamide at 180; for 6 h;Inert atmosphere;Sealed tube;Autoclave;chemoselective reaction;Reagent/catalyst;
Steps:
4.2. Preparation of mono- and difluorobenzyl chlorides. 4.2.1. General procedures. Method A.
General procedure: The reactions were carried out in a glass tube (V=10 mL) placed into a stainless steel autoclave (V=17 mL) at a controlled heating. The tube under argon was charged with FeCl2·4H2O (0.09 mmol, 0.018 g) and formamide (4.5 mmol, 0.20 g), the mixture was heated for 5 min, and then tetrachloromethane (18 mmol, 2.76 g), methanol (4.5 mmol, 0.14 g), and fluorotoluene 1-3 (9 mmol, 1.00 g) (or difluorotoluene 4-6 (9 mmol, 1.15 g)) were added. The sealed tube was placed into an autoclave, and the autoclave was sealed and heated for 6 h at 180 °C. After completion of the reaction, the autoclave was cooled down to room temperature, the tube was opened, and the reaction mixture was filtered through a paper filter. The solvent was distilled off. The target product was separated from the initial mono- or difluorotoluenes by vacuum distillation.
References:
Bayguzina, Alfiya R.;Gallyamova, Leysan I.;Khalilov, Leonard M.;Khusnutdinov, Ravil I. [Journal of Fluorine Chemistry,2019,vol. 226,art. no. 109346]
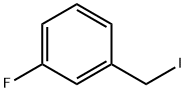
28490-56-4
47 suppliers
$348.00/10g

456-42-8
332 suppliers
$5.00/5g
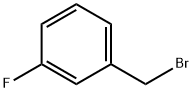
456-41-7
290 suppliers
$13.00/25g

456-42-8
332 suppliers
$5.00/5g
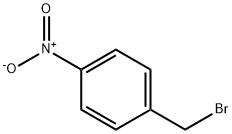
100-11-8
5 suppliers
$13.00/5g

456-42-8
332 suppliers
$5.00/5g
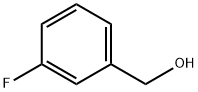
456-47-3
246 suppliers
$10.00/1g

456-42-8
332 suppliers
$5.00/5g