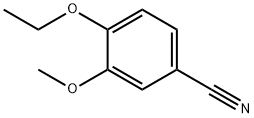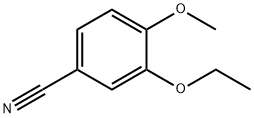
3-Ethoxy-4-methoxy benzonitrile synthesis
- Product Name:3-Ethoxy-4-methoxy benzonitrile
- CAS Number:60758-86-3
- Molecular formula:C10H11NO2
- Molecular Weight:177.2
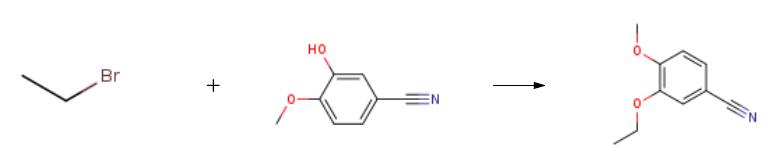
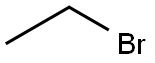
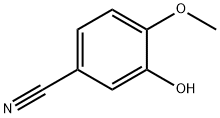
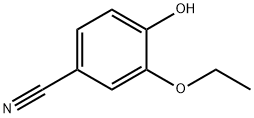
In a 100 ml flask in the, adding 3-hydroxy-4-methoxy phenyl nitrile 10g (67.11mmol), bromoethane 25 ml (335.2mmol), potassium carbonate 10.25g, dimethyl formamide 50 ml, heating and stirring to 100 °C. TLC monitoring reaction, reaction 8h, stop heating. Natural cooling to room temperature, water 100 ml, the ethyl acetate extraction, the organic phase is dried with anhydrous sodium sulfate, ethyl acetate solvent turns on lathe does the white solid obtained 11.09g.
1131-52-8
279 suppliers
$6.00/5g

60758-86-3
230 suppliers
$12.00/5g
Yield:60758-86-3 95.5%
Reaction Conditions:
with hydroxylamine hydrochloride in acetonitrile at 5 - 84;Product distribution / selectivity;
Steps:
5.1.1.1
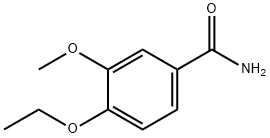
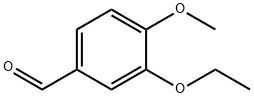
3-Ethoxy-4-methoxybenzaldehyde (1000 g, 5.54 moles, from Aldrich Chemicals,Milwaukee, WI) and hydroxylamine.HCl (462.5 g, 6.6 moles, from Aldrich Chemicals, Milwaukee, WI) were charged to a 12 L three-necked flask at room temperature, followed by the addition of acetonitrile (5 L, from Fisher Scientific, Pittsburgh, PA). The reaction mixture was stirred at room temperature for 15-20 minutes, and a latent endotherm (-5-15°C below room temperature) was observed. After the endotherm had subsided, the reaction mixture was warmed to 65-72°C. The reaction mixture was further heated to reflux at 78-84°C. After 2-3 hours of reflux, the reaction mixture was cooled to room temperature, and added with 1 L of deionized water. 3.5-4.0 L of acetonitrile from the reaction mixture was distilled off under vacuum. The concentrated residue was diluted with 4 L of deionized water, and stirred at room temperature for 1-2 hours. The mixture was then filtered at room temperature under vacuum. The filtered solid was washed with 3-4 L of deionized water. The solid was dried in a tray at 30-32°C for 24-36 hours under a pressure of 100-125 mm Hg. The yield of 3-ethoxy-4- methoxybenzonitrile was found to be 940 g (95.5%) based on 1000 g input of 3-ethoxy-4- methoxybenzaldehyde (HPLC indicated 99.2% purity by peak area).
References:
WO2010/30345,2010,A2 Location in patent:Page/Page column 10
247569-89-7
27 suppliers
inquiry

60758-86-3
230 suppliers
$12.00/5g