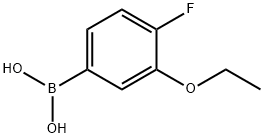
3-Ethoxy-4-fluorobenzeneboronic acid synthesis
- Product Name:3-Ethoxy-4-fluorobenzeneboronic acid
- CAS Number:900174-65-4
- Molecular formula:C8H10BFO3
- Molecular Weight:183.97
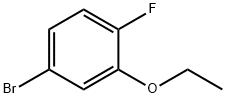
900174-64-3
28 suppliers
$60.00/50mg

900174-65-4
95 suppliers
$75.00/250 mg
Yield: 69%
Reaction Conditions:
Stage #1:4-Bromo-2-ethoxy-1-fluorobenzene with n-butyllithium in tetrahydrofuran;hexane at -78; for 0.666667 h;
Stage #2: with Trimethyl borate in tetrahydrofuran;hexane at -78 - 20; for 4 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;hexane;water
Steps:
9B
To a solution of 9A (3.86 g, 17.6 mmol) in THF (60 mL) at -78° C. was added n-BuLi (1.6 M in hexanes, 14.3 mL, 22.8 mmol). The mixture was stirred at -78° C. for 40 min before trimethyl borate (3.63 mL, 33 mmol) was added. The reaction was left stirring from -78° C. to rt over 4 h. It was quenched with 1.0 N HCl (40 mL), extracted with EtOAc, washed with brine and dried over Na2SO4. After evaporation of the solvent, the crude solid product was triturated with EtOAc/hexanes (1:4). After filtration, 9B (2.2 g, 69% yield) was collected as a white solid. 1H NMR (400 MHz, CDCl3) δ ppm 1.42 (t, J=7.03 Hz, 3H) 4.11 (q, J=7.03 Hz, 2H) 7.03 (dd, J=11.42, 8.35 Hz, 1H) 7.18-7.29 (m, 2H) 7.35 (d, J=7.91 Hz, 1H).
References:
Bristol-Myers Squibb Company US2010/227894, 2010, A1 Location in patent:Page/Page column 29