Yield:1201447-07-5 79%
Reaction Conditions:
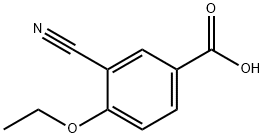
Stage #1: 3-cyano-4-(ethyloxy)benzoic acidwith benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in N,N-dimethyl-formamide at 20; for 0.333333 h;
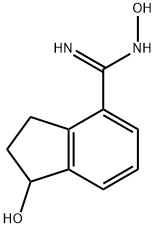
Stage #2: N',1-dihydroxy-2,3-dihydro-1H-indene-4-carboximidamide in N,N-dimethyl-formamide at 20 - 130; for 1.08333 h;Microwave irradiation;
Steps:
2-ethoxy-5-(3-(l-hydroxy-2,3-dihydro-lH-inden-4-yl)-l,2,4-oxadiazol-5- yl)benzonitrileIn a microwave vial, a stirring solution of 3-cyano-4-ethoxybenzoic acid (200mg, 1.05mmol) in DMF was treated with HOBt (185mg, 1.36mmol) and EDCI (260mg, 1.36mmol) at room temperature. The reaction was stirred for 20 min followed by addition, in a single portion, of iV,l-dihydroxy-2,3-dihydro-lH- indene-4-carboximidamide (261mg, 1.36mmol). The reaction was stirred for additional 30 min at room temperature and then heated to 130 °C for 35 min in the initiator. The reaction was diluted using a saturated solution of NaCl and extracted with EtOAc (80ml X3). The organic phase was dried over Na2SO4 anhydrous and concentrated under reduced pressure. The product was purified by column chromatography using CH2Cl2IMeOH (9:1) to offer the product as pale yellow solid in 79% yield (274mg).1H NMR (300 MHz, CDCl3-CH3OD): δ 8.31 (d, J= 2.1 Hz, IH), 8.25 (dd, Jl = 2.1 Hz, J2 = 8.7 Hz, IH), 7.96 (d, J= 7.5 Hz, IH), 7.46 (d, J= 7.5 Hz, IH), 7.29 (t, J= 7.8 Hz, IH), 7.06 (d, J= 9.0 Hz, IH), 5.16 (t, J= 6.0 Hz, IH), 4.18 (q, J = 6.9 Hz, 2H), 3.40-3.30 (m, IH), 3.10-2.93 (m, IH), 2.46-2.39 (m, IH), 1.94-1.87 (m, IH), 1.43 (t, J= 6.9 Hz, 3H); 13C NMR (75 MHz, CDCl3-CH3OD): δ 172.91, 167.05, 161.92, 145.72, 142.08, 133.24, 132.83, 127.38, 126.16, 126.10, 121.85, 115.98, 111.76, 101.85, 74.36, 64.55, 34.08, 30.18, 13.20. MS (EI) m/z 348 (M+), HRMS (EI) for C23H27N3O3 (M+): calcd 348.1343, found 348.1345.
References:
WO2009/151529,2009,A1 Location in patent:Page/Page column 167-168
![Benzonitrile, 5-[3-(2,3-dihydro-1-hydroxy-1H-inden-4-yl)-1,2,4-oxadiazol-5-yl]-2-ethoxy-](/CAS/20210305/GIF/1201447-07-5.gif)