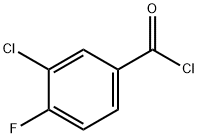
3-Chloro-4-fluorobenzoyl chloride synthesis
- Product Name:3-Chloro-4-fluorobenzoyl chloride
- CAS Number:65055-17-6
- Molecular formula:C7H3Cl2FO
- Molecular Weight:193
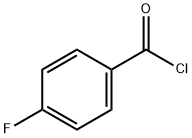
403-43-0
460 suppliers
$9.00/25g

65055-17-6
132 suppliers
$9.00/1g
Yield: 83.4%
Reaction Conditions:
with FeCl3
Steps:
1 EXAMPLE 1
EXAMPLE 1 100 g of 4-fluoro-benzoyl chloride and 0.5 g of FeCl3 are charged in a 250 ml flask. The mass is heated to a temperature of 80° C. and chlorine is fluxed at a rate of about 1 Nl/h. After 16 hours of reaction, the raw product is cooled, degassed with nitrogen and discharged. The product is vacuum distilled (15 mmHg) to separate the non converted reactant. 35 g of 4-fluoro-benzoyl chloride, 66 g of 3-chloro-4-fluoro-benzoyl chloride (I) and 10 g of impurities are obtained. Yield: 83.4%.
References:
Miteni S.p.A. US6066764, 2000, A
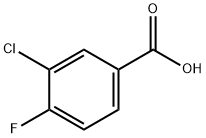
403-16-7
279 suppliers
$6.00/5g

65055-17-6
132 suppliers
$9.00/1g