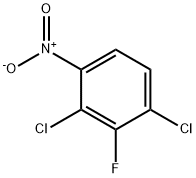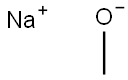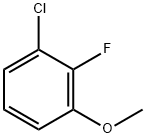
3-CHLORO-2-FLUOROANISOLE synthesis
- Product Name:3-CHLORO-2-FLUOROANISOLE
- CAS Number:261762-56-5
- Molecular formula:C7H6ClFO
- Molecular Weight:160.57
Yield: 112.4 g
Reaction Conditions:
Stage #1:1,3-dichloro-2-fluoro-4-nitrobenzene;sodium methylate in methanol at 40 - 50; for 2 h;
Stage #2: with Raney nickel catalyst in methanol at 40 - 50; under 7500.75 - 11251.1 Torr; for 4 h;Autoclave;Inert atmosphere;Further stages;Temperature;Reagent/catalyst;Solvent;
Steps:
1.1-1.3; 2-15
(1) Take a 2L four-necked flask and put in 500 mL of methanol and 210 g of 2,4-dichloro-3-fluoronitrobenzene in sequence, stir to dissolve, then heat to 40-50, and add 20% methanol with sodium methoxide dropwise 283.5g of solution, after dripping, heat preservation and reaction for 2h, sampling in control, HPLC: 2,4-dichloro-3-fluoronitrobenzene≤1%, 2-methoxy-3-fluoro-4-chloronitrobenzene And 2-chloro-3-fluoro-4-methoxynitrobenzene ≥95%, the reaction is over. Cool down to 20-30°C, add dropwise hydrochloric acid and methanol solution, adjust pH to about 7, and filter to remove the formed salt. (2) The mother liquor obtained in step (1) is put into a 2L autoclave, 4.2 g of Raney nickel catalyst is added, and the autoclave is closed. Replace with nitrogen three times and replace with hydrogen three times. The temperature is increased to 40-50°C, the pressure is controlled to 10-15bar, and the hydrogenation reaction is 4h until the hydrogen is no longer absorbed. Stop the hydrogenation, cool down to 20-30°C and evacuate, replace with nitrogen three times and discharge. The hydrogenation reaction liquid was filtered to recover Raney nickel, and the mother liquor was concentrated to dryness to obtain 158 g of a mixture of 2-methoxy-3-fluoro-4-chloroaniline and 2-chloro-3-fluoro-4-methoxyaniline , HPLC: ≥95%. (3) Put 158g of the mixture of 2-methoxy-3-fluoro-4-chloroaniline and 2-chloro-3-fluoro-4-methoxyaniline obtained in step (2) into a 2L four-neck flask, 20% Sulfuric acid 1500g, heated to all clear, slowly cooled to -5-5 , a large amount of white solid precipitated, and then began to slowly drip 144.9g 50% sodium nitrite solution, after the dripping, the solid was dissolved, and a small amount of sulfamic acid was added to eliminate Excessive sodium nitrite will not turn blue until the starch potassium iodide test paper is tested, and the diazonium solution is obtained. Take another 3L four-necked flask, put 96.8g of sodium hypophosphite, 31.7g of cuprous oxide, and 500g of 50% sulfuric acid, raise the temperature to 30-40°C, start dripping diazonium liquid, and nitrogen gas is released during the dripping process. After dripping, the reaction is incubated for 5 hours, sampling is controlled, HPLC: diazonium salt of 2-methoxy-3-fluoro-4-chloroaniline and 2-chloro-3-fluoro-4-methoxyaniline ≤1%, The reaction is over. Vapor distillation at elevated temperature, separation of the fractions, washing the oil layer with 20% sodium hydroxide solution 100g*2 twice, standing for separation to obtain 112.4g of 2-fluoro-3-chloroanisole, HPLC: ≥98%.
References:
Zhejiang Yongtai Technology Co., Ltd.;Shao Hongming;Zhang Xing;Yu Bo;Hu Jie;Li Hongkui CN112142567, 2020, A Location in patent:Paragraph 0041-0045; 0047-0048; 0050-0051; 0053-0054; 0056