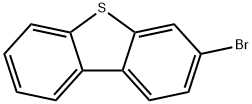
3-bromodibenzo[b,d]thiophene synthesis
- Product Name:3-bromodibenzo[b,d]thiophene
- CAS Number:97511-04-1
- Molecular formula:C12H7BrS
- Molecular Weight:263.15
![dibenzo[b,d]thiophen-3-aMine](/CAS/GIF/25288-76-0.gif)
25288-76-0
50 suppliers
inquiry
![3-bromodibenzo[b,d]thiophene](/CAS/20200331/GIF/97511-04-1.gif)
97511-04-1
109 suppliers
$16.00/250mg
Yield:97511-04-1 81%
Reaction Conditions:
Stage #1: 3-aminodibenzothiophenewith hydrogen bromide;sodium nitrite in water;
Stage #2: with sodium nitrite in water at 5;
Stage #3: with copper(I) bromide in water at 0 - 40; for 18 h;
Steps:
13.4 <4> > Synthesis of 3-bromodibenzothiophene
27.5 g (135.5 mmol) of the amino compound obtained in the above was placed in a 1 L four-necked round bottom flask equipped with a stirrer, a Liebig condenser (not required), a thermometer, and a 300 mL dropping funnel. , 250 mL of water and 75 mL of 48% hydrobromic acid were added and stirred overnight. Subsequently, the solution was cooled to -10 ° C. with an ice water bath, and a solution of 10.7 g (155.0 mmol) of sodium nitrite in 200 mL of water was added dropwise so that the temperature did not rise by 5 ° C. or more. Subsequently, the diazotized reaction solution was stirred at 5 ° C. or less for 1 hour to obtain a diazonium solution.Next, in a 1 L four-necked round bottom flask equipped with a stirrer, Erlin condenser, thermometer, and 1 L dropping funnel, 21.4 g (154.4 mmol) of cuprous bromide, 48% hydrobromic acid 50 mL and 120 mL of water were placed and cooled to 0 ° C. and stirred. Subsequently, the diazonium solution was added dropwise at a temperature not exceeding 5 ° C., and the mixture was stirred at the same temperature for 30 minutes, further heated to 40 ° C. and stirred for 18 hours.To the obtained reaction solution, 260 mL of water was added, cooled to room temperature, and extracted twice with DCM 260 mL. Next, the organic layer was washed with 130 mL of a 5% sodium sulfite aqueous solution, further washed with 130 mL of saturated brine, and then dried with magnesium sulfate. Subsequently, the magnesium sulfate was removed by suction filtration, and the solvent was distilled off under reduced pressure. Subsequently, the resulting crude product was purified by silica gel column chromatography using n-heptane as a developing solution to obtain 28.9 g (yield: 81.1%) of the objective bromide
References:
JP2018/90561,2018,A Location in patent:Paragraph 0078
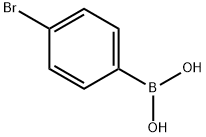
5467-74-3
465 suppliers
$8.00/1g
![3-bromodibenzo[b,d]thiophene](/CAS/20200331/GIF/97511-04-1.gif)
97511-04-1
109 suppliers
$16.00/250mg
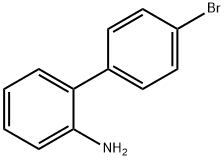
62532-98-3
18 suppliers
inquiry
![3-bromodibenzo[b,d]thiophene](/CAS/20200331/GIF/97511-04-1.gif)
97511-04-1
109 suppliers
$16.00/250mg
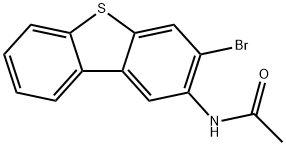
65642-89-9
1 suppliers
inquiry
![3-bromodibenzo[b,d]thiophene](/CAS/20200331/GIF/97511-04-1.gif)
97511-04-1
109 suppliers
$16.00/250mg
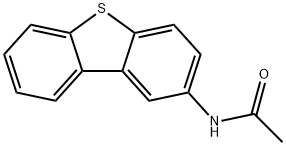
54818-88-1
2 suppliers
$28.60/10mg
![3-bromodibenzo[b,d]thiophene](/CAS/20200331/GIF/97511-04-1.gif)
97511-04-1
109 suppliers
$16.00/250mg