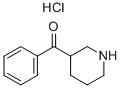
3-BENZOYLPIPERIDINE HYDROCHLORIDE synthesis
- Product Name:3-BENZOYLPIPERIDINE HYDROCHLORIDE
- CAS Number:5562-52-7
- Molecular formula:C12H15NO.ClH
- Molecular Weight:225.717
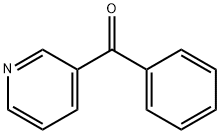
5424-19-1
195 suppliers
$20.00/10g

5562-52-7
17 suppliers
$64.00/250mg
Yield:5562-52-7 96%
Reaction Conditions:
Stage #1: 3-Benzoylpyridinewith N,N,N,N,N,N-hexamethylphosphoric triamide in dichloromethane at 25; for 0.166667 h;Inert atmosphere;
Stage #2: with trichlorosilane in dichloromethane at 25; for 24 h;Catalytic behavior;Inert atmosphere;Solvent;Temperature;
Steps:
3.1. Reduction of Pyridines and N-Heteroaromatics
General procedure: Under an argon atmosphere, pyridine 1 or N-heteroaromatic 3 (0.10 mmol) and HMPA (3.5 mg,0.02 mmol) were added in anhydrous DCM (0.7 mL) and stirred at room temperature for 10 min, andthen trichlorosilane (2.0 M, 0.3 mL) was added. The reaction was stirred at room temperature for 24h, quenched with H2O, and then the pH was adjusted to ~7-8 with saturated NaHCO3. The mixturewas extracted with EtOAc (3 × 5 mL). The combined organic layers were washed with brine, driedover anhydrous Na2SO4, concentrated under reduced pressure and purified with columnchromatography (silica gel, DCM/MeOH/TEA = 10/1/0.1) to afford a pure product. 3-Benzoylpiperidin-1-ium Chloride (2a). Colorless oil. 1H-NMR (400 MHz, CDCl3): δ 7.97-7.95 (m, 2H),7.58-7.55 (m, 1H), 7.49-7.45 (m, 2H), 3.64-3.46 (m, 1H), 3.27-3.24 (m, 3H), 3.10 (d, J = 12.3 Hz, 1H),2.90 (dd, J = 12.4, 9.9 Hz, 1H), 2.71-2.69 (m, 1H), 2.04-2.02 (m, 1H), 1.84-1.56 (m, 3H). 13C-NMR (101MHz, CDCl3): δ 202.4, 136.0, 133.1, 128.7, 128.3, 48.9, 46.3, 44.7, 28.0, 25.4. HRMS (+ESI) m/z calculatedfor [M + H]+ 190.1226, found 190.1230.
References:
Fu, Yun;Sun, Jian [Molecules,2019,vol. 24,# 3,art. no. 401]
884510-91-2
1 suppliers
inquiry

5562-52-7
17 suppliers
$64.00/250mg
![1-Boc-3-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/189442-78-2.gif)
189442-78-2
80 suppliers
$10.00/100mg

5562-52-7
17 suppliers
$64.00/250mg