3-benzhydrylpyridine synthesis
- Product Name:3-benzhydrylpyridine
- CAS Number:3678-71-5
- Molecular formula:C18H15N
- Molecular Weight:245.32
Yield:3678-71-5 70%
Reaction Conditions:
with [1,1′-biphenyl]-2-yldiphenylphosphane;ammonium acetate;ammonium formate;palladium diacetate;caesium carbonate in tert-Amyl alcohol at 90; for 4 h;Inert atmosphere;Schlenk technique;
Steps:
Palladium-Catalyzed Reductive Coupling Reaction for the Synthesisof Triarylmethanes; {[4-(tert-Butyl)phenyl]methylene}dibenzene(6a);7c Typical Procedure 4
General procedure: Under an argon atmosphere, Pd(OAc)2 (3.3 mg, 0.015 mmol, 5 mol%),ligand [1,1′-biphenyl]-2-yldiphenylphosphane (15 mg, 0.045 mmol,15 mol%), HCO2NH4 (23 mg, 0.36 mmol), MeCO2NH4 (35 mg, 0.45mmol), benzopheneone N-tosylhydrazone 5a (126 mg, 0.36 mmol),and Cs2CO3 (195 mg, 0.6 mmol) were successively added to a flamedried flamedried25 mL Schlenk flask. The reaction flask was degassed threetimes with argon and anhyd tert-pentyl alcohol (3.0 mL) was addedusing a syringe. Then 1-bromo-4-(tert-butyl)benzene (2b; 64 mg, 0.3mmol) was added with a syringe. Note that the aryl bromide in a solidform was added to the reaction flask prior to Cs2CO3. The reactionwas heated at 90 °C with stirring for about 4 h, then it was cooled tor.t., and filtered through a short pad of neutral alumina with EtOAc(25 mL) as eluent. Solvent was then removed in vacuo to leave a crudemixture, which was purified by silica gel column chromatography(eluent: PE); yellow solid (82 mg, 91%).
References:
Xia, Yamu;Hu, Fangdong;Xia, Ying;Liu, Zhenxing;Ye, Fei;Zhang, Yan;Wang, Jianbo [Synthesis,2017,vol. 49,# 5,art. no. SS-2016-H0531-OP,p. 1073 - 1086]
19490-91-6
9 suppliers
inquiry

3678-71-5
6 suppliers
$28.60/1g
620-95-1
94 suppliers
$45.00/250mg
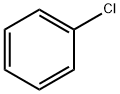
108-90-7
655 suppliers
$10.00/25G

3678-71-5
6 suppliers
$28.60/1g
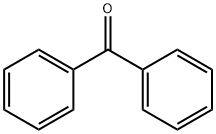
119-61-9
796 suppliers
$5.00/10g

3678-71-5
6 suppliers
$28.60/1g
![Benzene, 1,1'-[(phenylsulfonyl)methylene]bis-](/CAS/20210305/GIF/5433-76-1.gif)