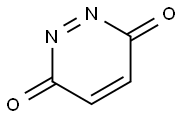
3,6-Pyridazinedione synthesis
- Product Name:3,6-Pyridazinedione
- CAS Number:42413-70-7
- Molecular formula:C4H2N2O2
- Molecular Weight:110.07
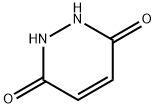
123-33-1
420 suppliers
$11.76/25G

42413-70-7
18 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 3,6-dihydroxypyridazinewith potassium hydroxide in water;
Stage #2: with tert-butylhypochlorite in acetone at -78; for 3 h;Inert atmosphere;
Steps:
Synthesis of 9.
Synthesis of 3,6-dihydroxypyridazine potassium salt. A suspension of 3,6-dihydroxypyridazine (1.00 g, 8.92 mmol) in water (4.0 mL) was treated with potassium hydroxide (0.50 g, 8.91 mmol). The reaction mixture was stirred until the solution became clear. The solvent was removed under reduced pressure and the achieved 3, 6-dihydroxypyridazine potassium salt was finely pestled and dried in an exsiccator (drying agent: phosphorus pentoxide); To a stirred suspension of 3,6-dihydroxypyridazine potassium salt (0.31 g, 2.10 mmol) and acetone (15 mL) was added tert-butylhypochlorite solution (0.24 mL, 2.10 mmol) at -78 °C. After stirring for 3 h a solution of diene 3 (0.55 g, 2.00 mmol) and acetone (5 mL) was added. The reaction mixture was slowly warmed at r.t., stirred for further 1 h and then quenched with water (50 mL). The volatile organic components were evaporated and the resulting aqueous residue was extracted with diethyl ether (3 × 20 mL), the combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The product was purified by silica column chromatography (ethyl acetate) to yield 0.15 g (20%) of 9 as a pale yellow solid.
References:
Mayer, Christoph D.;Bracher, Franz [European Journal of Medicinal Chemistry,2011,vol. 46,# 8,p. 3227 - 3236] Location in patent:experimental part
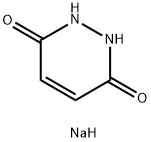
28330-26-9
3 suppliers
inquiry

42413-70-7
18 suppliers
inquiry
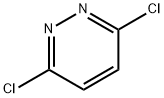
141-30-0
399 suppliers
$6.00/10g

42413-70-7
18 suppliers
inquiry

123-33-1
420 suppliers
$11.76/25G

42413-70-7
18 suppliers
inquiry