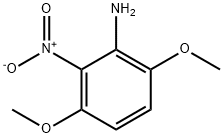
3,6-Dimethoxy-2-nitroaniline synthesis
- Product Name:3,6-Dimethoxy-2-nitroaniline
- CAS Number:26002-57-3
- Molecular formula:C8H10N2O4
- Molecular Weight:198.18
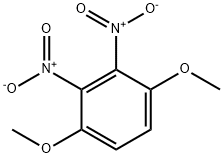
6945-76-2
11 suppliers
inquiry

26002-57-3
3 suppliers
inquiry
Yield:26002-57-3 95%
Reaction Conditions:
Stage #1: 2,3-dinitro-1,4-dimethoxybenzenewith iron(III) trichloride hexahydrate in methanol at 65; for 1 h;
Stage #2: with hydrazine hydrate monohydrate in methanol at 65; for 1 h;
Steps:
4.1.5. Synthesis of 3,6-dimethoxy-2-nitroaniline (6)
A mixture of 1,4-dimethoxy-2,3-dinitrobenzene (4.56 g, 20 mmol) (1), activated charcoal (0.8 g), iron (III) chloride hexahydrate (324.4 mg, 1.2 mmol) in methanol (25 mL), was stirred at 65°C for 1 h. Then, hydrazine monohydrate (3.84 mL, 79 mmol) was added drop wise and stirred at 65°C for 1 h. The reaction was allowed to cool to room temperature and the solids were filtered and washed with methanol. The filtrate was dried (MgSO4), concentrated and the residue was purified by column chromatography (ethyl acetate/hexane, 1:1) to give 6 as an orange solid (3.76 g, 95%), mp 68-69°C (lit. [28] 67-68°C).
References:
López-Lira, Claudia;Tapia, Ricardo A.;Herrera, Alejandra;Lapier, Michel;Maya, Juan D.;Soto-Delgado, Jorge;Oliver, Allen G.;Graham Lappin;Uriarte, Eugenio [Bioorganic Chemistry,2021,vol. 111,art. no. 104823]
150-78-7
523 suppliers
$5.00/25g

26002-57-3
3 suppliers
inquiry
110-86-1
852 suppliers
$9.00/5g
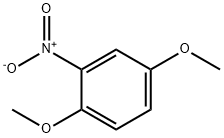
89-39-4
104 suppliers
$46.00/5g
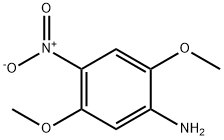
6313-37-7
106 suppliers
$21.00/1g

26002-57-3
3 suppliers
inquiry
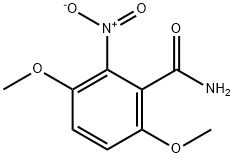
26002-58-4
3 suppliers
inquiry

26002-57-3
3 suppliers
inquiry