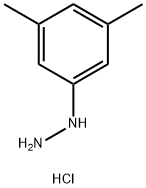
3,5-Dimethylphenylhydrazine hydrochloride synthesis
- Product Name:3,5-Dimethylphenylhydrazine hydrochloride
- CAS Number:60481-36-9
- Molecular formula:C8H13ClN2
- Molecular Weight:172.66
108-69-0
252 suppliers
$9.00/1g

60481-36-9
149 suppliers
$22.00/1g
Yield: 85.22%
Reaction Conditions:
Stage #1:3,5-dimethylaminoaniline with hydrogenchloride in water at 25 - 35; for 0.333333 h;
Stage #2: with sodium nitrite in water at -5 - 0;
Stage #3: with hydrogenchloride;tin(II) chloride dihdyrate in water at -5 - 35;
Steps:
7a
Preparation of 3,5-Dimethyl phenyl hydrazine hydrochloride 2220.0 ml hydrochloric acid was charged into a 5.0 L 4 necked round bottom flask connected to a mechanical stirrer, thermo meter socket, and condenser. 200.0 g (1.65 mol) of 3,5-Dimethyl aniline was charged at 25-35° C. Reaction mass was stirred for 20 min. Reaction mass was cooled to -5 to 0° C. Sodium nitrite solution [120.0 g (1.74 mol) of Sodium nitrite was dissolved in 1060.0 ml of DM Water and cooled to 0-5° C.) was added to dimethyl aniline mass at -5 to 0° C. for 60-90 min. Maintained the mass temperature at -5 to 0° C. for 60-75 min. 740.0 ml of hydrochloric acid was charged into a 10.0 L 4 necked round bottom flask connected to a mechanical stirrer, thermo meter socket, and condenser. 746.0 g of stannous chloride.2H2O (3.30 mol) was charged. Stirred the mass for 30-45 min at 25-35° C. Reaction mass was cooled to -5 to 0° C. The diazotized solution was added slowly to stannous chloride solution at -5 to 0° C. for 150-180 min. Maintained the mass temperature at -5 to 0° C. for 30-45 min. Reaction mass temperature was raised to 25-35° C. Maintained the mass temperature at 25-35° C. for 90-120 min. Solid was filtered and solid was washed with 200.0 ml of water. Compound was dried under vacuum at 55-60° C. 1000.0 ml of ethanol and crude compound were charged into a 2.0 L 4 necked round bottom flask connected to a mechanical stirrer, thermo meter socket, and condenser. Raised the mass temperature to reflux temperature. Maintained the mass temperature at reflux for 30-45 min. 20.0 g of carbon was charged and maintained the mass temperature at reflux for 30-45 min. Carbon was filtered and carbon was washed with 200.0 ml of ethanol. Collected the filtrate into a flask. Distilled off ethanol completely under vacuum at mass temperature not crossing 60° C. Mass temperature was cooled to 25-35° C. and release the vacuum. 800.0 ml of isopropyl ether was charged. Maintained the mass temperature at 25-35° C. for 45-60 min and mass temperature was cooled to 0 to 5° C. Maintained the mass temperature at 0 to 5° C. for 45-60 min. Solid was filtered and solid was washed with 200.0 ml of isopropyl ether. Compound was dried under vacuum at 45-50° C. till obtaining constant weight. Dry weight of the compound weight: 243.0 g (yield 85.22%) Purity by HPLC: 99.6%, 3.5-Dimethyl content by HPLC is 0.13% Spectral data: FT-IR (K Br) (cm-1): 3237, 3118, 3008, 2919, 2662,1608, 1588, 1577, 1533, 1518, 1308, 1276, 1159,1061, 684, cm-1. 400 MHz 1H NMR (DMSO-d6): δ value (ppm): 2.20 s (2-CH3), 2.49 s (NH2), 6.58 s (Ar-Ha, Hb, Hc. 8.10 broad (NH), 10.09 broad (HCl).13C NMR δ value: 21.11(2C), 112.33(2C), 123.02(1C), 137.98(2C), 145.56(1C).Mass: 137.26 [M]+1, -HCl, 121.20 [M-NH2]
References:
Natco Pharma Limited US2010/298351, 2010, A1 Location in patent:Page/Page column 31
556-96-7
381 suppliers
$6.00/5g

60481-36-9
149 suppliers
$22.00/1g