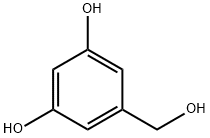
3,5-Dihydroxybenzyl alcohol synthesis
- Product Name:3,5-Dihydroxybenzyl alcohol
- CAS Number:29654-55-5
- Molecular formula:C7H8O3
- Molecular Weight:140.14
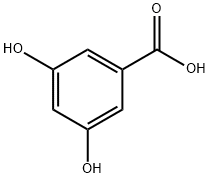
99-10-5
519 suppliers
$5.00/5g

29654-55-5
296 suppliers
$10.00/1g
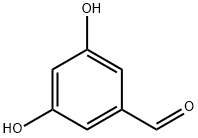
Yield:29654-55-5 85%
Reaction Conditions:
with sodium tetrahydroborate;Trimethyl borate;dimethyl sulfate in tetrahydrofuran at 20; for 5.5 h;
Steps:
1.1 A method for synthesizing 3,5-dihydroxybenzaldehyde, comprising the following steps
step 1,Under the protection of nitrogen,Dimethyl sulfate (2 kg) was added dropwise to a solution of sodium borohydride (615 g) in tetrahydrofuran (1 kg) at 0 ° C for 1 hour.Stirring at 0 ° C for 1 hour, then stirring at room temperature for 4 hours until the mixture was free of gas;Further, a solution of 3,5-dihydroxybenzoic acid (1 kg) and trimethyl borate (2 kg) in tetrahydrofuran (1 kg) was added dropwise thereto, and the mixture was added dropwise for 0.5 hour, and stirred at room temperature for 5 hours;After the reaction is over, keep the system at 0 °C.Deionized water (1 kg) was added thereto and stirred for 0.5 hours.Tetrahydrofuran was distilled off at 50 ° C using a rotary evaporator, followed by extraction with ethyl acetate (2 kg).Then, wash back three times with saturated sodium bicarbonate solution (2 kg each time), and wash the backwash 3 times with saturated brine (2 kg each time).Finally, it was dried and concentrated at 45 ° C using a rotary evaporator.Obtained white solid B (770 g), yield 85%
References:
CN110256214,2019,A Location in patent:Paragraph 0030-0037
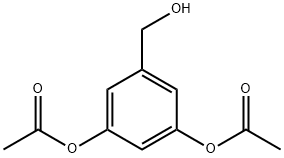
35354-30-4
0 suppliers
inquiry

29654-55-5
296 suppliers
$10.00/1g
26153-38-8
259 suppliers
$20.00/250mg

29654-55-5
296 suppliers
$10.00/1g
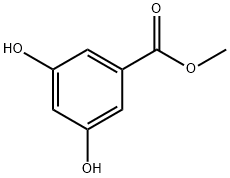
2150-44-9
261 suppliers
$5.00/5g

29654-55-5
296 suppliers
$10.00/1g
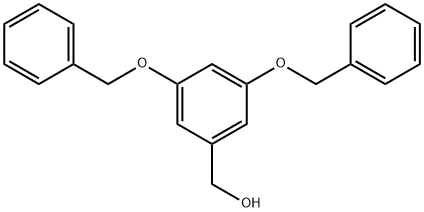
24131-31-5
158 suppliers
$32.00/1g

29654-55-5
296 suppliers
$10.00/1g