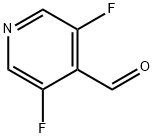
3,5-Difluoro-4-pyridinecarboxaldehyde synthesis
- Product Name:3,5-Difluoro-4-pyridinecarboxaldehyde
- CAS Number:870234-98-3
- Molecular formula:C6H3F2NO
- Molecular Weight:143.09
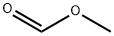
107-31-3
343 suppliers
$16.00/25mL
71902-33-5
166 suppliers
$6.00/1g

870234-98-3
84 suppliers
$57.00/100mg
Yield:870234-98-3 81%
Reaction Conditions:
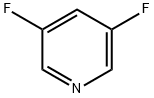
Stage #1:3,5-difluoropyridine with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -70; for 0.5 h;Inert atmosphere;
Stage #2:Methyl formate in tetrahydrofuran;hexane at -70; for 1.5 h;
Steps:
113A 3,5-Difluoroisonicotinaldehyde
Example 113A
3,5-Difluoroisonicotinaldehyde
Under argon and at -70° C., 44 ml of 2.5 M n-butyllithium solution in n-hexane (110 mmol, 1.1 equivalent) were slowly added dropwise to 15.4 ml of diisopropylamine (110 mmol, 1.1 equivalent) in 23 ml of THF.
The solution formed was warmed to 0° C. and stirred at this temperature for 30 min.
The reaction mixture was then brought to -70° C. and diluted with 23 ml of THF, and 11.5 g of 3,5-difluoropyridine (100 mmol, 1 equivalent) dissolved in 72 ml of THF, were added dropwise.
The mixture was stirred at -70° C. for 30 min. 12.4 ml of methyl formate (200 mmol, 2 equivalent), dissolved in 23 ml of THF, were then slowly added dropwise.
After 1.5 h at -70° C., the reaction solution was quickly poured into 230 ml of saturated aqueous sodium bicarbonate solution and extracted with a total of 460 ml of ethyl acetate.
The combined organic phases were washed twice with in each case 115 ml of saturated aqueous sodium bicarbonate solution and twice with saturated aqueous sodium chloride solution, dried over sodium sulphate and concentrated using a rotary evaporator.
This gave 11.6 g (81% of theory) of the title compound which were directly reacted further.
GC-MS (Method 14): Rt=1.82 min
MS (ESpos): m/z=144.0 (M+H)+
1H-NMR (400 MHz, DMSO-d6): δ=8.75 (br. s, 2H), 10.24 (br. s, 1H).
References:
BAYER PHARMA AKTIENGESELLSCHAFT;VAKALOPOULOS, Alexandros;HARTUNG, Ingo;FOLLMANN, Markus;JAUTELAT, Rolf;STRAUB, Alexander;HA?FELD, Jorma;LINDNER, Niels;SCHNEIDER, Dirk;WUNDER, Frank;STASCH, Johannes-Peter;REDLICH, Gorden;LI, Volkhart Min-Jian;BECKER-PELSTER, Eva Maria;KNORR, Andreas US2014/128386, 2014, A1 Location in patent:Paragraph 1410; 1411; 1412; 1413; 1414

71902-33-5
166 suppliers
$6.00/1g
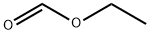
109-94-4
477 suppliers
$10.00/10g

870234-98-3
84 suppliers
$57.00/100mg
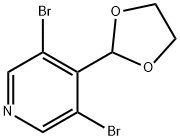
924649-13-8
7 suppliers
inquiry

870234-98-3
84 suppliers
$57.00/100mg
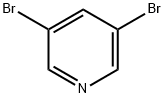
625-92-3
493 suppliers
$6.00/10g

870234-98-3
84 suppliers
$57.00/100mg
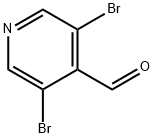
70201-42-2
209 suppliers
$5.00/1g

870234-98-3
84 suppliers
$57.00/100mg