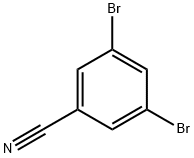
3,5-DIBROMOBENZONITRILE synthesis
- Product Name:3,5-DIBROMOBENZONITRILE
- CAS Number:97165-77-0
- Molecular formula:C7H3Br2N
- Molecular Weight:260.91
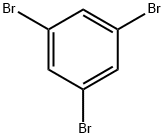
626-39-1
467 suppliers
$5.00/5g

97165-77-0
163 suppliers
$7.00/1g
Yield:97165-77-0 75%
Reaction Conditions:
Stage #1:1,3,5-trisbromobenzene with isopropylmagnesium chloride;lithium chloride in tetrahydrofuran at -15; for 0.25 h;
Stage #2: with N,N-dimethyl-formamide in tetrahydrofuran at 0; for 2 h;
Stage #3: with ammonia;iodine in tetrahydrofuran;water at 20; for 2 h;
Steps:
3.28 Typical experimental procedure for conversion of aromaticbromides into aromatic nitriles with iPrMgCl, DMF, I2,and aq NH3
General procedure: To a flask containing dried LiCl (0.35 g, 8.24 mmol) was added iPrMgCl (2 M in THF, 4.1 mL) and THF (5 mL) at 15° C. After beingstirred for 15 min, 3-bromo-1-benzonitrile (1.46 g, 8.03 mmol) inTHF (1 mL) was added to the reaction mixture and the obtainedmixture was stirred for 15 min. Then, DMF (1.3 mL, 12 mmol) wasadded at 0° C and the mixture was stirred for 2 h. Then, aq NH3 (7 mL, 28-30%) and I2 (4.06 g, 16 mmol) were added to the reaction mixture. After being stirred for 2 h at room temperature, the reactionmixture was poured into satd aq Na2SO3 solution and was extracted with CHCl3 (3?30 mL). The organic layer was dried over Na2SO4 and filtered. After removal of the solvent, the residue waspurified by short column chromatography on silica gel (eluent:hexane/ethyl acetate=9:1, v/v) to provide pure 1,3-dicyanobenzene (0.73 g) in 71% yield. Most nitriles mentioned in this work are commercially availableand were identified by comparison with the authentic samples.
References:
Ishii, Genki;Harigae, Ryo;Moriyama, Katsuhiko;Togo, Hideo [Tetrahedron,2013,vol. 69,# 5,p. 1462 - 1469]
145691-59-4
113 suppliers
$6.00/100mg
175205-85-3
61 suppliers
$25.00/1g

97165-77-0
163 suppliers
$7.00/1g
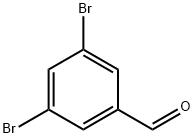
56990-02-4
285 suppliers
$6.00/1g

97165-77-0
163 suppliers
$7.00/1g

175205-85-3
61 suppliers
$25.00/1g

97165-77-0
163 suppliers
$7.00/1g
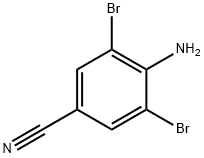
58633-04-8
68 suppliers
$6.00/250mg

97165-77-0
163 suppliers
$7.00/1g