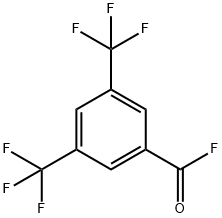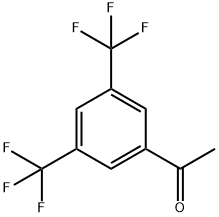
3',5'-Bis(trifluoromethyl)acetophenone synthesis
- Product Name:3',5'-Bis(trifluoromethyl)acetophenone
- CAS Number:30071-93-3
- Molecular formula:C10H6F6O
- Molecular Weight:256.14
328-70-1
415 suppliers
$10.00/25 g

30071-93-3
373 suppliers
$10.00/10 g
Yield:30071-93-3 82%
Reaction Conditions:
with acetic anhydride;magnesium in tetrahydrofuran;water
Steps:
3 1-(3,5-Bis(trifluoromethyl)phenyl)ethan-1-one
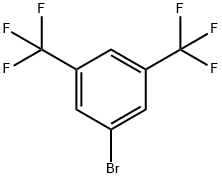
EXAMPLE 3 1-(3,5-Bis(trifluoromethyl)phenyl)ethan-1-one A solution of 3,5-Bis(trifluoromethyl)bromobenzene (29.3 g) in 30 mL of THF was added to a mixture of magnesium granules (5.10 g) in THF (200 mL) heated at reflux (the reaction was initiated with approximately 5 mL of the bromide solution; the remainder was added slowly over 1 h). The mixture was aged for 30 min at reflux, cooled to RT and added over 1 h to a solution of acetic anhydride (40 mL) in THF (40 mL) maintained at -15° C. The resulting dark brown mixture was warmed to 10° C. in a water bath, and water (300 mL) was added. The pH of the vigorously stirred biphasic mixture was adjusted to 8.0 using 50% NaOH. MTBE (300 mL) was added, the layers were separated and the aqueous layer was further extracted with MTBE (3*150 mL). The organic layers were combined and concentrated in vacuo (bath at 30-35° C.; 50-80 torr). The concentrate was then distilled at atmospheric pressure to provide the pure product (20.7 g; 82% yield) with a boiling point of 187-189° C.
References:
McNamara, James M.;Zhao, Matthew M. US2002/22725, 2002, A1 Brands, Karel M. Jos;Tsay, Fuh-Rong;Conrad, Karen M.;Zhao, Matthew M. US2002/52494, 2002, A1

328-70-1
415 suppliers
$10.00/25 g
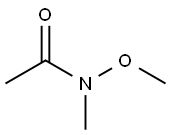
78191-00-1
279 suppliers
$6.00/5g

30071-93-3
373 suppliers
$10.00/10 g

328-70-1
415 suppliers
$10.00/25 g
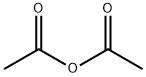
108-24-7
5 suppliers
$14.00/250ML

30071-93-3
373 suppliers
$10.00/10 g

328-70-1
415 suppliers
$10.00/25 g
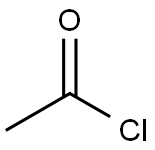
75-36-5
579 suppliers
$17.92/100G

30071-93-3
373 suppliers
$10.00/10 g