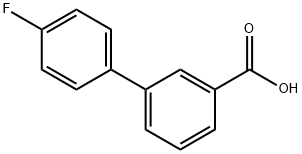
3-(4-FLUOROPHENYL)BENZOIC ACID synthesis
- Product Name:3-(4-FLUOROPHENYL)BENZOIC ACID
- CAS Number:10540-39-3
- Molecular formula:C13H9FO2
- Molecular Weight:216.21
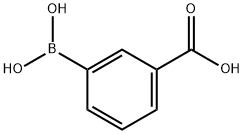
25487-66-5
471 suppliers
$6.00/5g
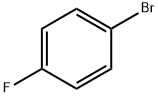
460-00-4
622 suppliers
$6.00/10g

10540-39-3
110 suppliers
$14.00/250mg
Yield: 86%
Reaction Conditions:
with tetrabutylammomium bromide;sodium carbonate in water at 100; for 5 h;Suzuki Coupling;
Steps:
3.D1 Step D1
General procedure: The required aryl bromide, 3-carboxyphenylboronic acid (1.05 eq), Na2CO3 (2 eq), TBAB (0.01 eq) are given in a screw-cap reactor. H2O (2 mL/mmol aryl bromide) and 10 mM Pd(EDTA) solution (0.3 mL/mmol aryl bromide) are added, the reactor is closed tightly and heated to 100°C under stirring. After 5 hours the mixture is cooled to room temperature, diluted with AcOEt (10 mL/mmol aryl bromide) and extracted with 5 % NaHCO3 (4x10 mL/mmol aryl bromide). The pooled basic extracts are acidified to pH 3 by dropwise addition of concentrated HCI under stirring, then extracted with AcOEt (3x 10 mL/mmol aryl bromide). The pooled organic extracts are washed with H2O (10 mL/mmol aryl bromide) and brine (10 mL/mmol aryl bromide), dried over Na2SO4, filtered and evaporated to dryness under reduced pressure. If necessary, the crude product is purified by preparative-HPLC.
References:
DSM IP ASSETS B.V.;BOUDON, Stéphanie;GEOTTI-BIANCHINI, Piero;HEIDL, Marc;JACKSON, Eileen;SCHLIFKE-POSCHALKO, Alexander WO2017/12890, 2017, A1 Location in patent:Page/Page column 22; 24
585-76-2
351 suppliers
$6.00/5g
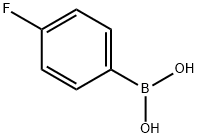
1765-93-1
558 suppliers
$9.00/1g

10540-39-3
110 suppliers
$14.00/250mg

1765-93-1
558 suppliers
$9.00/1g
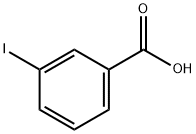
618-51-9
387 suppliers
$9.00/10g

10540-39-3
110 suppliers
$14.00/250mg
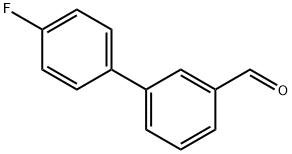
164334-74-1
81 suppliers
$29.00/100mg

10540-39-3
110 suppliers
$14.00/250mg
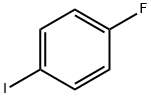
352-34-1
368 suppliers
$7.00/5g

10540-39-3
110 suppliers
$14.00/250mg