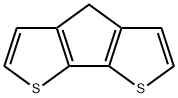
3,4-Dithia-7H-cyclopenta[a]pentalene synthesis
- Product Name:3,4-Dithia-7H-cyclopenta[a]pentalene
- CAS Number:389-58-2
- Molecular formula:C9H6S2
- Molecular Weight:178.27
![4H-Cyclopenta[2,1-b:3,4-b']dithiophen-4-one](/CAS/GIF/25796-77-4.gif)
25796-77-4
154 suppliers
$22.00/100mg
![3,4-Dithia-7H-cyclopenta[a]pentalene](/CAS/GIF/389-58-2.gif)
389-58-2
200 suppliers
$24.00/100mg
Yield:389-58-2 66%
Reaction Conditions:
with potassium hydroxide;hydrazine in ethylene glycol at 190; for 13 h;
Steps:
1
References:
EP1997821,2008,A1 Location in patent:Page/Page column 32
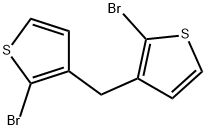
17965-52-5
0 suppliers
inquiry
![3,4-Dithia-7H-cyclopenta[a]pentalene](/CAS/GIF/389-58-2.gif)
389-58-2
200 suppliers
$24.00/100mg
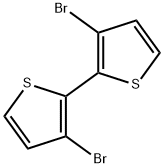
51751-44-1
239 suppliers
$7.00/100mg
![Bis[(pinacolato)boryl]Methane](/CAS/GIF/78782-17-9.gif)
78782-17-9
99 suppliers
$22.00/1g
![3,4-Dithia-7H-cyclopenta[a]pentalene](/CAS/GIF/389-58-2.gif)
389-58-2
200 suppliers
$24.00/100mg
![4H-Cyclopenta[2,1-b:3,4-b']dithiophene-4-carboxylic acid](/CAS/20210305/GIF/78196-93-7.gif)
78196-93-7
0 suppliers
inquiry
![3,4-Dithia-7H-cyclopenta[a]pentalene](/CAS/GIF/389-58-2.gif)
389-58-2
200 suppliers
$24.00/100mg
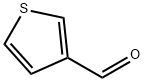
498-62-4
407 suppliers
$5.00/1g
![3,4-Dithia-7H-cyclopenta[a]pentalene](/CAS/GIF/389-58-2.gif)
389-58-2
200 suppliers
$24.00/100mg