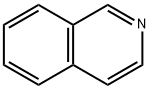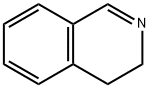
3,4-DIHYDROISOQUINOLINE synthesis
- Product Name:3,4-DIHYDROISOQUINOLINE
- CAS Number:3230-65-7
- Molecular formula:C9H9N
- Molecular Weight:131.17
Yield:-
Reaction Conditions:
with sodium hydroxide in dichloromethane
Steps:
P.17.b Production Example 17b
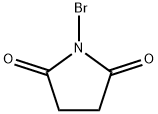
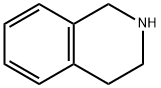
Production Example 17b 3,4-Dihydroisoquinoline N-Bromosuccinimide (39.2 g) was added to a methylene chloride solution (300 ml) containing 26.67 g (0.2 mol) of 1,2,3,4-tetrahydroisoquinoline under ice-cooling over 20 minutes. After stirring for 40 minutes, an aqueous 30% sodium hydroxide solution (130 ml) was added to the reaction solution. The organic layer was washed with water and then extracted with a 10% aqueous hydrochloric acid (200 ml). The aqueous layerwas washed with methylene chloride, basified with an aqueous ammonia, and then extracted with methylene chloride. The extract was dried over magnesium sulfate and then evaporated. The resulting residue was distilled (about 16 mmHg, 120°C), to give 21.5 g of the title compound as an oil. 1H-NMR(DMSO-d6) δ (ppm): 2.66 (2H, t, J=8 Hz), 3.62 (2H, td, J= 2 Hz, 8 Hz), 7.19-7.21 (1H, m), 7.29-7.33 (1H, m), 7.35-7.40 (1H, m), 8.31 (1H, t, J= 2 Hz).
References:
Eisai Co., Ltd. EP1258252, 2002, A1
91-21-4
254 suppliers
$5.00/5g

3230-65-7
78 suppliers
$29.00/100mg
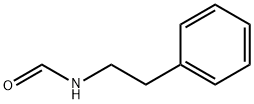
23069-99-0
27 suppliers
$56.20/1g

3230-65-7
78 suppliers
$29.00/100mg
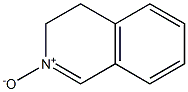
24423-87-8
0 suppliers
inquiry

3230-65-7
78 suppliers
$29.00/100mg