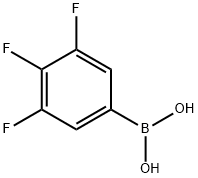
3,4,5-Trifluorophenylboronic acid synthesis
- Product Name:3,4,5-Trifluorophenylboronic acid
- CAS Number:143418-49-9
- Molecular formula:C6H4BF3O2
- Molecular Weight:175.9
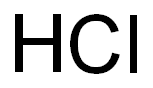
7647-01-0
11 suppliers
$10.00/10g
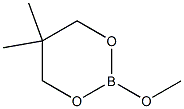
57186-57-9
0 suppliers
inquiry
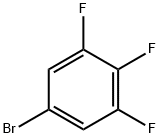
138526-69-9
416 suppliers
$6.00/5g

143418-49-9
398 suppliers
$5.00/1g
Yield:143418-49-9 90.3%
Reaction Conditions:
Stage #1: 5-bromo-1,2,3-trifluorobenzenewith iodine;magnesium in 2-methyltetrahydrofuran at 40 - 50;
Stage #2: 2-methoxy-5,5,-dimethy-1,3,2-dioxaborinane in 2-methyltetrahydrofuran at 20 - 30; for 4 h;
Stage #3: hydrogenchloride in 2-methyltetrahydrofuran;water monomer; for 1.5 h;Solvent;Temperature;
Steps:
5-6 Example 5
Add 48mmol (11.7g) of magnesium scraps into a 1000mL four-necked flask, add 2 iodine, then add 200mL 2-methyltetrahydrofuran, after heating the temperature to 50°C, slowly let it cool down to 40°C naturally, then Add dropwise 5 mL of a mixed solution consisting of 84.4 g (0.4 mol) of 3,4,5-trifluorobromobenzene/50 mL of 2-methyltetrahydrofuran to initiate a Grignard reaction. After the reaction is initiated, the temperature will rise rapidly. The temperature was lowered to 20-30°C, and a mixed solution (1) consisting of 84.4 g (0.4 mol) of 3,4,5-trifluorobromobenzene and 50 mL of 2-methyltetrahydrofuran was added dropwise, and a mixture consisting of methoxyneopentylglycol boron 80.6 g (0.56 mol) of the acid ester and 50 mL of 2-methyltetrahydrofuran constitute a mixed solution (2). The speed of dropping the mixed solution (1) and the mixed solution (2) was kept the same, the dropping speed was controlled, and the dropping temperature was kept at 20-30 °C. After the dropping was completed, the reaction was continued at room temperature for 4 h.After the reaction was completed, the reaction solution was slowly poured into dilute hydrochloric acid, stirred for 1.5 h after the dropwise addition, and the liquids were separated. The aqueous phase was extracted twice, the organic phases were combined, and dried over anhydrous sodium sulfate. Spin dry and recrystallize from dichloromethane to obtain a white powder with a yield of 90.3%, a content of 99.4%, and related impurities below 200 ppm.
References:
CN114149460,2022,A Location in patent:Paragraph 0040-0045
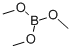
121-43-7
358 suppliers
$14.00/25mL

138526-69-9
416 suppliers
$6.00/5g

143418-49-9
398 suppliers
$5.00/1g
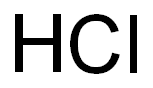
7647-01-0
11 suppliers
$10.00/10g
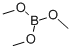
121-43-7
358 suppliers
$14.00/25mL

138526-69-9
416 suppliers
$6.00/5g

143418-49-9
398 suppliers
$5.00/1g
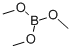
121-43-7
358 suppliers
$14.00/25mL
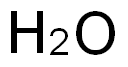
7732-18-5
469 suppliers
$13.50/100ML

138526-69-9
416 suppliers
$6.00/5g

143418-49-9
398 suppliers
$5.00/1g
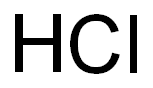
7647-01-0
11 suppliers
$10.00/10g
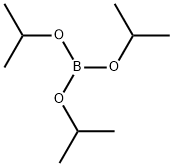
5419-55-6
377 suppliers
$12.00/5g

138526-69-9
416 suppliers
$6.00/5g

143418-49-9
398 suppliers
$5.00/1g