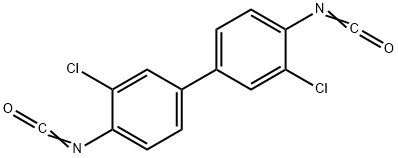
3,3'-DICHLORODIPHENYL 4,4'-DIISOCYANATE synthesis
- Product Name:3,3'-DICHLORODIPHENYL 4,4'-DIISOCYANATE
- CAS Number:5331-87-3
- Molecular formula:C14H6Cl2N2O2
- Molecular Weight:305.12
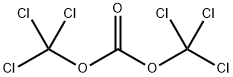
32315-10-9
414 suppliers
$10.00/1g
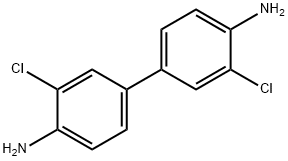
91-94-1
93 suppliers
$30.00/1g

5331-87-3
27 suppliers
$110.00/500mg
Yield:-
Reaction Conditions:
with N-1-octyl morpholine in dichloromethane;lithium hydroxide monohydrate; for 1 h;Flow reactor;
Steps:
42
General procedure: Triphosgene Solution: A triphosgene solution (triphosgene concentration: 0.0661 M) obtained by dissolving triphosgene in methylene chloride was prepared. Tertiary Amine Solution: An N-(2-ethylhexyl)morpholine solution (N-(2-ethylhexyl)morpholine concentration: 0.529M) obtained by dissolving N-(2-ethylhexyl)morpholine in methylene chloride was prepared. Active Hydrogen-Containing Compound Solution: A phenethylamine solution (phenethylamine concentration: 0.132 M) obtained by dissolving phenethylamine in methylene chloride was prepared. Liquid Feeding Conditions: Triphosgene solution: 1.0 mL/min Tertiary amine solution: 1.0 mL/min Active hydrogen-containing compound solution: 1.0 mL/min The water content of the solvent of each of the above solutions is shown in the table below. Purity of Reaction Product (Isocyanate Compound): A reaction solution was collected from the outlet port (most downstream) of the flow channel (V), diluted 500-fold with a reaction solvent (methylene chloride in Example 1), and the diluted sample was analyzed by gas chromatography under the following conditions to measure the purity. The results are shown in the table below. In the table below, “>97” means that the purity is more than 97% by mass, and “<10” means that the purity is less than 10% by mass. A pressure gauge was installed in the middle of the flow channel between the tertiary amine solution introduction port (iB) and the joining part (M2) (that is, inside the flow channel (II)), and the pressure after 1 hour passed at the time when the liquid feeding became stable and the reaction was in a steady state, was evaluated as evaluation “A” in a case of less than 0.05 MPa, as evaluation “B” in a case of 0.05 MPa or more and less than 0.1 MPa, and as evaluation “C” in a case of 0.1 MPa or more. The results are shown in the table below.
References:
US2022/135517,2022,A1 Location in patent:Paragraph 0149; 0164-0186; 0193