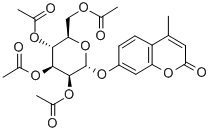
4-Methylumbelliferyl2,3,4,6-tetra-O-acetyl-a-D-mannopyranoside synthesis
- Product Name:4-Methylumbelliferyl2,3,4,6-tetra-O-acetyl-a-D-mannopyranoside
- CAS Number:28541-71-1
- Molecular formula:C24H26O12
- Molecular Weight:506.46
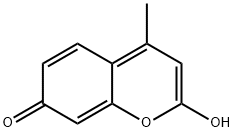
79566-13-5
6 suppliers
inquiry
4163-65-9
194 suppliers
$15.00/1g

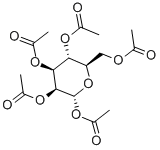
28541-71-1
46 suppliers
$250.00/250mg
Yield:28541-71-1 59%
Reaction Conditions:
with pyridine;boron trifluoride diethyl etherate in 1,2-dichloro-ethane at 60; for 5 h;Inert atmosphere;
Steps:
12 Example 12
A mixture of 4-MU (1.057 g, 6.00 mmol, 1.0 eq) and α/β-D-mannopose pentaacetate (1.171 g, 3.00 mmol, 0.5 eq) of dry ClCH2CH2Cl under argon ( In a suspension of 9 ml), dry pyridine (728 μL, 9.00 mmol, 1.5 eq), BF3.OEt2 (5792 μL, 45.00 mmol, 7.5 eq) were added successively, and the reaction was stirred at 60 ° C for 5 h, then diluted with 9 ml of CH 2 Cl 2 and then used. The reaction is stopped in a Na2CO3 solution or a saturated NaHCO3 solution, followed by washing with a dilute NaOH solution until the color of the solution becomes light or alkaline, and then washed with water and saturated brine successively, and the organic phase is dried with anhydrous Na2SO4, and separated. The organic phase was evaporated to remove the solvent, followed by recrystallization from anhydrous ethanol three times.After drying, white and yellowish needle-like crystals were obtained 0.899g(Yield 59%, labeled as Compound 10, which has the structure shown in Formula 10)
References:
CN104926898,2018,B Location in patent:Paragraph 0063; 0064