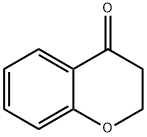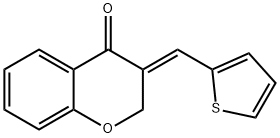
3-[(E)-2-THIENYLMETHYLIDENE]-2,3-DIHYDRO-4H-CHROMEN-4-ONE synthesis
- Product Name:3-[(E)-2-THIENYLMETHYLIDENE]-2,3-DIHYDRO-4H-CHROMEN-4-ONE
- CAS Number:256485-62-8
- Molecular formula:C14H10O2S
- Molecular Weight:242.29
Yield:256485-62-8 85%
Reaction Conditions:
with pyrrolidine in methanol at 20; for 3 h;
Steps:
17 4.1.2. General procedure for the synthesis of (E)-3-heteroarylidenechroman-4-ones (1a-r)
General procedure: METHOD A: Pyrrolidine (10 mmol) was added to a mixture of the appropriate chroman-4-one (6.7 mmol) and heteroaryl aldehyde (10 mmol) in dry MeOH (15 mL for 1a, 1b, 1e, 1h, 1k-r and 30mL for 1c, 1d, 1i, 1j). The reaction mixture was stirred at room temperature for 1-24 h (1h for 1f, 3h for 1l-q, 24h for 1a-c, 1h-j, 1n, and 1o). The mixture was diluted with ice-water, and the precipitate was filtered off and washed with water. The crude solid was purified by column chromatography on silica gel, eluting with a mixture of AcOEt and CHCl3 (3:1) (1a-g and 1i-r) or CHCl3 (1h) and crystallized from n-hexane. METHOD B: (synthesis of 1f and 1g) A mixture of chroman-4-one (6.7 mmol), the appropriate pyridinecarbaldehyde (10 mmol) and pyrrolidine (10 mmol) was heated at 120 °C under stirring for 1 h. After cooling, the mixture was diluted with ice-water, and the precipitate was filtered off and washed with water. The crude solid was purified by column chromatography on silica gel, eluting with a mixture of AcOEt and CHCl3 (3:1), and crystallized from n-hexane. 4.1.2.17
(E)-3-[(Thiophen-2-yl)methylene]chroman-4-one (1q)
Yield: 85%, mp = 126-127 °C (lit. 125-126 °C)
[16]
. IR (KBr): 1661 cm-1. 1H NMR (CDCl3, 400 MHz): δ (ppm) 8.01 (d, 1H, H5, J5-6 = 7.2 Hz), 7.98 (s, 1H, =CH), 7.59 (d, 1H, H3', J3'-4' = 4.4 Hz), 7.48 (t, 1H, H7, J7-8 = J7-6 = 7.2 Hz), 7.34 (d, 1H, H5', J4'-5' = 4.4 Hz), 7.16 (t, 1H, H4', J3'-4' = J4'-5' = 4.4 Hz), 7.06 (t, 1H, H6, J5-6 = J7-6 7.2 Hz), 6.98 (d, 1H, H8, J7-8 = 7.2 Hz), 5.46 (d, 2H, H2, Jall = 1.2 Hz).
13C NMR (CDCl3, 100 MHz): δ (ppm) 181.24, 161.02, 137.67, 135.69, 133.88, 130.94, 128.70, 128.20, 127.87, 127.28, 121.95, 121.89, 117.83, 67.81. Anal. Calcd. for C14H10O2S: C, 69.40; H, 4.16; S, 13.23. Found: C, 69.26; H, 4.16; S, 13.28.
References:
Desideri, Nicoletta;Proietti Monaco, Luca;Fioravanti, Rossella;Biava, Mariangela;Yá?ez, Matilde;Alcaro, Stefano;Ortuso, Francesco [European Journal of Medicinal Chemistry,2016,vol. 117,p. 292 - 300]
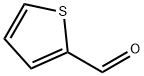
98-03-3
556 suppliers
$5.00/25g
![3-[(E)-2-THIENYLMETHYLIDENE]-2,3-DIHYDRO-4H-CHROMEN-4-ONE](/StructureFile/ChemBookStructure2/GIF/CB9772203.gif)
256485-62-8
6 suppliers
inquiry
393148-57-7
6 suppliers
inquiry
![3-[(E)-2-THIENYLMETHYLIDENE]-2,3-DIHYDRO-4H-CHROMEN-4-ONE](/StructureFile/ChemBookStructure2/GIF/CB9772203.gif)
256485-62-8
6 suppliers
inquiry
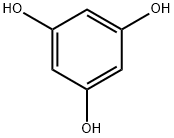
108-73-6
509 suppliers
$13.00/5g
![3-[(E)-2-THIENYLMETHYLIDENE]-2,3-DIHYDRO-4H-CHROMEN-4-ONE](/StructureFile/ChemBookStructure2/GIF/CB9772203.gif)
256485-62-8
6 suppliers
inquiry