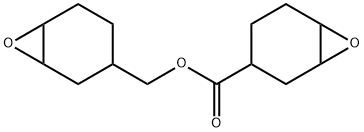
3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate synthesis
- Product Name:3,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate
- CAS Number:2386-87-0
- Molecular formula:C14H20O4
- Molecular Weight:252.31
2611-00-9
88 suppliers
inquiry

2386-87-0
297 suppliers
$8.00/10g
Yield:2386-87-0 94%
Reaction Conditions:
with phosphotungstic acid;trioctylmethylammonium acetate;phosphoric acid;dihydrogen peroxide;sodium carbonate in water;toluene at 50; for 15 h;Inert atmosphere;
Steps:
2
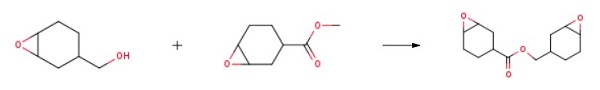
mixer,Reflux condenser,In a flask equipped with a stirrer,12 parts of water was added while purging with nitrogen,0.38 parts of 12-tungstophosphoric acid,0.56 parts of phosphoric acid,Sodium carbonate was added,The pH was adjusted to 5.0.Further, 0.6 part of trioctylmethylammonium acetate (xylene solution of trioctylmethylammonium acetate manufactured by Lion Akzo) was added,After refining the tungstic acid type catalyst,35 parts of toluene was added and dissolved,As a two-layer system solution,The mixture was stirred vigorously at room temperature for 1 hour.Here, equation (1).And 22 parts of the compound represented by the formulaThe solution was heated to 50 ° C.,While stirring,24.8 parts of 30% by weight hydrogen peroxide water was added,The mixture was further stirred at 50 ° C. for 15 hours as a postreaction.After cooling to room temperature,1 part of 30% by weight sodium hydroxide aqueous solution,10 parts of 20% by weight aqueous sodium thiosulfate solution was added and stirred for 1 hour,After 15 parts of toluene was further added,It was left standing.after that,The organic layer separated into two layers was taken out.To the obtained organic layer, 8.8 parts of Hokuetsu HS (phenol · formaldehyde resin beads manufactured by Ajinomoto Fine-Techno Co., Ltd.) which had been washed with methanol in advance was added,The mixture was stirred at room temperature for 2 hours and filtered under reduced pressure.The resulting solution was washed three times with 30 parts of water,Using a rotary evaporator,By distilling off the organic solvent,20 parts of a desired epoxy compound (EP1) was obtained.The obtained epoxy compound was pale yellow,Epoxy equivalent is 130 g / eq. , And the viscosity was 241 mPa · s.The amount of remaining quaternary ammonium salt (gas chromatography) was 50 ppm,The residual tungsten content (ashing method) was 1 ppm.The obtained epoxy compound contained 6% monoepoxy compound,93% diepoxy compound,It is 1% water adduct,Main purity measurement by GPC showed 99%.
References:
NIPPON KAYAKU COMPANY LIMITED;NAKANISHI, MASATAKA JP5748191, 2015, B2 Location in patent:Paragraph 0063