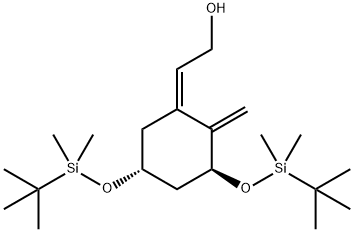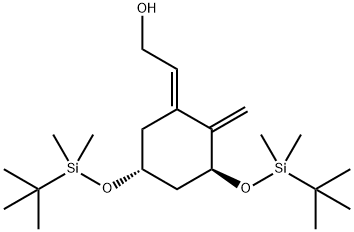
(E)-2-((3S,5R)-3,5-bis((tert-butyldimethylsilyl)oxy)-2-methylenecyclohexylidene)ethan-1-ol synthesis
- Product Name:(E)-2-((3S,5R)-3,5-bis((tert-butyldimethylsilyl)oxy)-2-methylenecyclohexylidene)ethan-1-ol
- CAS Number:227961-42-4
- Molecular formula:C21H42O3Si2
- Molecular Weight:398.73
![Ethanol, 2-[(3S,5R)-3,5-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2-methylenecyclohexylidene]-, 1-acetate, (2E)-](/CAS/20210305/GIF/318248-17-8.gif)
318248-17-8
0 suppliers
inquiry

227961-42-4
3 suppliers
inquiry
Yield:227961-42-4 201 mg
Reaction Conditions:
with methanol;potassium carbonate in dichloromethane at 20; for 0.5 h;Inert atmosphere;
Steps:
4.3.1 (E)-2-[(3S,5R)-3,5-Bis{(tert-butyldimethylsilyl)oxy}-2-methylenecyclohexylidene]ethan-1-ol (21)
To a solution of 20 (470 mg, 1.18 mmol) in CH2Cl2 (5 mL) were added triethylamine (329 μL, 2.36 mmol), acetic anhydride (181 μL, 1.77 mmol), and then dimethylaminopyridine (7.2 mg, 0.059 mmol) at 0 °C and stirred at room temperature for 45 min. After the reaction was quenched by NH4Cl at 0 °C, the mixture was extracted with AcOEt, washed with brine, dried over Na2SO4, filtered and concentrated. The residue was purified by short column chromatography on neutral silica gel (hexane/AcOEt=50/1-10/1) to give a crude product, which was used without further separation. To a solution of a part of the above product (21 mg, 0.0476 mmol) in dry THF (2 mL) were added triethylamine (100 μL, 0.714 mmol), triphenylphosphine (12.5 mg, 0.0476 mmol), and then Pd(OAc)2 (5.3 mg, 0.0238 mmol) at room temperature, and stirred at 50 °C. After 24 h, the reaction mixture was concentrated, and the residue was purified by short column chromatography on neutral silica gel (hexane/AcOEt=50/1-40/1) to give a crude product, which was used without further separation. To a solution of a part of the product (10.5 mg, 0.0238 mmol) in CH2Cl2 (1 mL) and MeOH (1 mL) were added K2CO3 (7 mg, 0.036 mmol) at 0 °C and stirred at room temperature for 30 min. After the reaction was quenched by NH4Cl at 0 °C, the mixture was extracted with AcOEt, washed with brine, dried over Na2SO4, filtered and concentrated. The residue was purified by flash column chromatography on silica gel (hexane/AcOEt=10/1-8/1) to give 2116 (201 mg, 88% in 2 steps) as a colorless oil.
References:
Sawada, Daisuke;Kakuda, Shinji;Kamimura-Takimoto, Midori;Takeuchi, Akiko;Matsumoto, Yotaro;Kittaka, Atsushi [Tetrahedron,2016,vol. 72,# 22,p. 2838 - 2848]
![Acetic acid, 2-[(3S,5R)-3,5-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2-methylenecyclohexylidene]-, ethyl ester, (2E)-](/CAS/20210305/GIF/81506-23-2.gif)
81506-23-2
0 suppliers
inquiry

227961-42-4
3 suppliers
inquiry
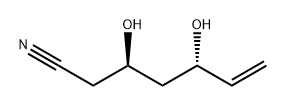
342644-77-3
0 suppliers
inquiry

227961-42-4
3 suppliers
inquiry
![6-Heptenenitrile, 3,5-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, (3R,5S)-](/CAS/20210305/GIF/342645-69-6.gif)
342645-69-6
0 suppliers
inquiry

227961-42-4
3 suppliers
inquiry
![Methanesulfonic acid, 1,1,1-trifluoro-, (1Z,3S,5S)-3,5-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]-1-(2-hydroxyethylidene)-6-hepten-1-yl ester](/CAS/20210305/GIF/342645-98-1.gif)