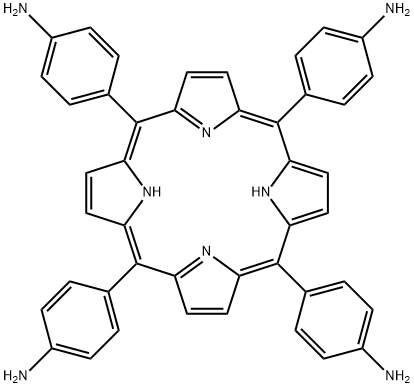
5,10,15,20-TETRAKIS(4-AMINOPHENYL)-21H,23H-PORPHINE synthesis
- Product Name:5,10,15,20-TETRAKIS(4-AMINOPHENYL)-21H,23H-PORPHINE
- CAS Number:22112-84-1
- Molecular formula:C44H34N8
- Molecular Weight:674.79
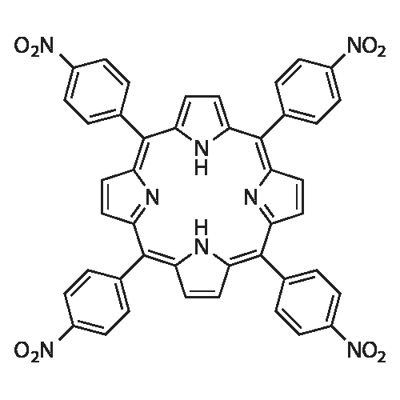
22843-73-8
53 suppliers
inquiry

22112-84-1
160 suppliers
$60.00/100mg
Yield:22112-84-1 94%
Reaction Conditions:
Stage #1:5,10,15,20-tetrakis(4-nitrophenyl)porphyrin with hydrogenchloride in water for 0.5 h;Inert atmosphere;
Stage #2: with tin(II) chloride dihdyrate in water at 80; for 0.5 h;Inert atmosphere;
Steps:
5,10,15,20-tetrakis(4-aminophenyl)porphyrin (2d)
A mixture of p-nitrobenzaldehyde (11.3 g, 75 mmol), acetic anhydride (14 mL, 151 mmol) andpropionic acid (200 mL) was placed into a 500 mL three necked round-bottom flask fitted withmechanical stirrer, reflux condenser, thermometer. Then the reaction mixture was heated to theboiling point, to which pyrrole (5.2 mL, 75 mmol) was added. The mixture was refluxed for 30min. It was then cooled to room temperature. The resulting precipitate was obtained by filtrationand washed with DMF and hot water. The residue was recrystallized from pyridine to give5,10,15,20-trakis(4-nitrophenyl)porphyrin (1d) (12.1 g, 24% yield).Compound 1d (12.1 g, 15.23 mmol) was dissolved in concentrated hydrochloric acid (600 mL)and stirred for 30 min. Tin(II) dichloride dehydrate (51.5 g, 228 mmol) was added. The mixturewas heated to 80 °C for 30 min. Water (200 mL) was poured into the reaction, then cooled to room temperature and stand for 24 h. The resulting green precipitate was obtained by filtration.The precipitate was dissolved in water (200 mL). The green solution was neutralized withammonium hydroxide until the color changed to violet. The resulting violet precipitate wasobtained by filtration and washed with water. The product was purified by columnchromatography (silica gel, CH2Cl2/MeOH, 60:1) to give the compound 2d (9.65 g, 94% yield).
References:
Meng, Shuai;Xu, Zengping;Hong, Ge;Zhao, Lihui;Zhao, Zhanjuan;Guo, Jianghong;Ji, Haiying;Liu, Tianjun [European Journal of Medicinal Chemistry,2015,vol. 92,p. 35 - 48] Location in patent:supporting information
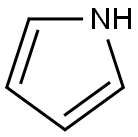
109-97-7
345 suppliers
$9.00/5 g
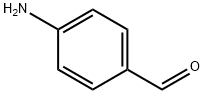
556-18-3
192 suppliers
$89.00/5g

22112-84-1
160 suppliers
$60.00/100mg
67595-98-6
12 suppliers
inquiry

22112-84-1
160 suppliers
$60.00/100mg
555-16-8
543 suppliers
$5.00/5g

22112-84-1
160 suppliers
$60.00/100mg

109-97-7
345 suppliers
$9.00/5 g

555-16-8
543 suppliers
$5.00/5g

22112-84-1
160 suppliers
$60.00/100mg