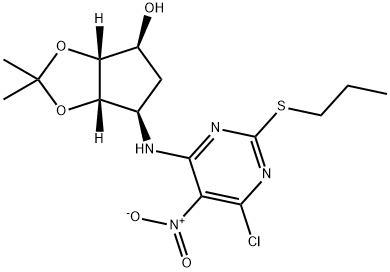
(3aR,4S,6R,6aS)-6-[[6-Chloro-5-nitro-2-(propylthio)-4-pyrimidinyl]amino]tetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-ol synthesis
- Product Name:(3aR,4S,6R,6aS)-6-[[6-Chloro-5-nitro-2-(propylthio)-4-pyrimidinyl]amino]tetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-ol
- CAS Number:220241-59-8
- Molecular formula:C15H21ClN4O5S
- Molecular Weight:404.87
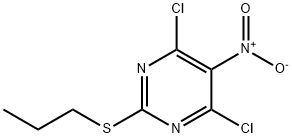
145783-14-8
261 suppliers
$11.00/1g
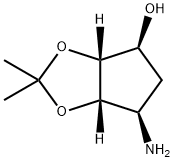
155899-66-4
222 suppliers
$20.00/1g
![(3aR,4S,6R,6aS)-6-[[6-Chloro-5-nitro-2-(propylthio)-4-pyrimidinyl]amino]tetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-ol](/CAS/20150408/GIF/220241-59-8.gif)
220241-59-8
14 suppliers
inquiry
Yield:220241-59-8 84%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20; for 1 h;
Steps:
2 Example 2
Preparation of (3aR,4S,6R,6aS)-6-((6-chloro-5-nitro-2-(propylthio)pyrimidin-4-yl)amino)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ol (AMALCIN)
The title compound was prepared using the method described in .
To a solution of CLIN (11.6 g, 43.3 mmol) and Et3N (4.0 mL, 28.9 mmol) in dry THF (100 mL) solution of AMAL (5.0 g, 28.9 mmol) in dry THF (100 mL) was slowly added at room temperature, and resulting reaction mixture was stirred for 1 h.
Salts were filtered off, washed with dry THF (50 mL), filtrate was concentrated, and crude product was purified by crystallization from hexane/EtOAc mixture to afford yellowish powder (m = 9.88 g, 84% yield). MP 63°C; 1H NMR (DMSO-d6, 500 MHz) δ 0.98 (t, J = 7.3 Hz, 3H), 1.19 (s, 3H), 1.34 (s, 3H), 1.64-1.78 (m, 3H), 2.17 (m, 1H), 3.04 (m, 1H), 3.12 (m, 1H), 4.11 (m, 1H), 4.44 (m, 1H), 4.53-4.58 (m, 2H), 8.76 (br d, J = 7.9 Hz, 1H); MS (ESI) m/z: 405 [MH]+.
References:
EP2586773,2013,A1 Location in patent:Paragraph 0061-0063

145783-14-8
261 suppliers
$11.00/1g
220329-21-5
4 suppliers
inquiry
![(3aR,4S,6R,6aS)-6-[[6-Chloro-5-nitro-2-(propylthio)-4-pyrimidinyl]amino]tetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-dioxol-4-ol](/CAS/20150408/GIF/220241-59-8.gif)
220241-59-8
14 suppliers
inquiry