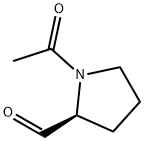
2-Pyrrolidinecarboxaldehyde, 1-acetyl-, (S)- (9CI) synthesis
- Product Name:2-Pyrrolidinecarboxaldehyde, 1-acetyl-, (S)- (9CI)
- CAS Number:73323-64-5
- Molecular formula:C7H11NO2
- Molecular Weight:141.17
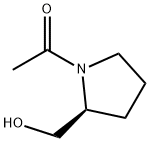
66158-68-7
17 suppliers
$474.00/500mg

73323-64-5
12 suppliers
$764.00/1g
Yield:73323-64-5 85.5%
Reaction Conditions:
Stage #1: (S)-1-(2-(hydroxymethyl) pyrrolidin-1-yl)ethanonewith oxalyl dichloride in dichloromethane at -78; for 0.5 h;Inert atmosphere;
Stage #2: with triethylamine in dichloromethane;Inert atmosphere;
Steps:
681.B (S)-1-acetylpyrrolidine-2-carbaldehyde
A solution of oxalyl dichloride (0.683 g, 5.38 mmol) in dry DCM (20 mL) was cooled to -78 °C under a N2atmosphere and(methylsulfinyl)methane (0.840 g, 10.8 mmol) was added to the reaction mixture, followed by (5 -1-(2-(hydroxymethyl)pyrrolidin-l -yl)ethanone (0.700 g, 4.89 mmol) and was stirred for 30 minutes at -78 °C. Triethylamine (2.473 g, 24.44 mmol) was added dropwise and the reaction was allowed to warm up to room temperature. After complete consumption of (S)--(2- (hydroxymethyl)pyrrolidin-1-yl)ethanone, the reaction was quenched with distilled water, stirred for 5 minutes and the reaction mixture was extracted DCM. The combined organic extracts were dried over anhydrous Na2SC>4 and concentrated to dryness to give the title compound (0.590 g, 85.5% yield).
References:
WO2017/100668,2017,A1 Location in patent:Page/Page column 644
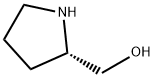
23356-96-9
467 suppliers
$11.19/5G

73323-64-5
12 suppliers
$764.00/1g