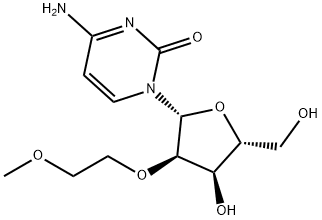
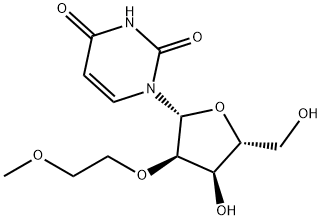
2'-O-(2-Methoxyethyl)cytidine synthesis
- Product Name:2'-O-(2-Methoxyethyl)cytidine
- CAS Number:223777-16-0
- Molecular formula:C12H19N3O6
- Molecular Weight:301.3
223777-15-9
113 suppliers
$19.00/100mg

223777-16-0
99 suppliers
inquiry
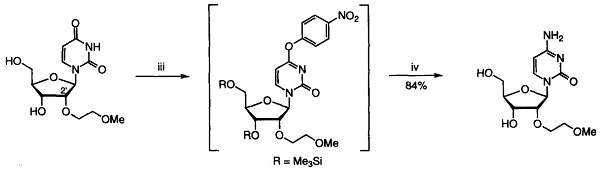
Yield:223777-16-0 84%
Reaction Conditions:
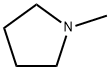
Stage #1: 2'-O-(2-methoxyethyl)uridinewith 1-Methylpyrrolidine;chloro-trimethyl-silane in acetonitrile at 20; for 1 h;
Stage #2: with trifluoroacetic anhydride in acetonitrile at 0; for 0.0833333 h;
Stage #3: with 4-nitro-phenol;sodium bicarbonate;ammonia;watermore than 3 stages;
Steps:
Preparation of 2'-O-(2-Methoxyethyl)cytidine
Preparation of 2'-O-(2-Methoxyethyl)cytidine 2'-O-(2-Methoxyethyl)uridine (6.05g, 20.0mmol), 1-methylpyrrolidine (20ml, 0.192mol), chlorotrimethylsilane (7.6ml, 59.9mmol) and dry acetonitrile (100ml) were stirred together at room temperature. After 1 hour, the reactants were cooled to 0°C (ice-water bath) and trifluoroacetic anhydride (7.1ml, 50.3 mmol) was added dropwise over 5 minutes. After a further period of 30 minutes at 0°C, 4-nitrophenol (8.35g, 60mmol) was added to the stirred reactants which were maintained at 0°C. After 3 hours, the products were poured into saturated aqueous sodium hydrogencarbonate (200ml), and the resulting mixture was extracted with dichloromethane (3 x 100ml). The combined organic layers were dried (MgSO4), and evaporated under reduced pressure. Concentrated aqueous ammonia (d 0.88, 20ml) was added to a stirred solution of the residue in dioxane (100ml), contained in a sealed flask that was then heated at 55°C for 24 hours. The resulting yellow solution was concentrated under reduced pressure, and the residue was evaporated with absolute ethanol (3 x 50ml). The products were fractionated by short column chromatography on silica gel: the appropriate fractions, which were eluted with dichloromethane-methanol-triethylamine (93:7:0.5 to 90:10:0.5 v/v) were evaporated under the reduced pressure to give the title compound as an off-white solid (5.07g 84%).
References:
EP1165584,2004,B1 Location in patent:Page 5
120-94-5
404 suppliers
$8.00/25g
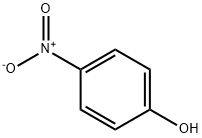
100-02-7
18 suppliers
$11.00/5G

223777-15-9
113 suppliers
$19.00/100mg

223777-16-0
99 suppliers
inquiry
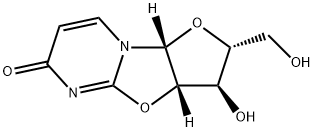
3736-77-4
373 suppliers
$5.00/100mg

223777-16-0
99 suppliers
inquiry
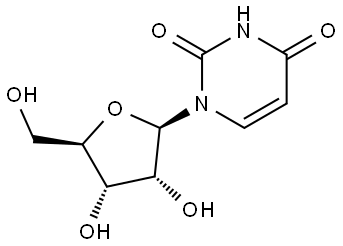
58-96-8
656 suppliers
$5.00/1g

223777-16-0
99 suppliers
inquiry