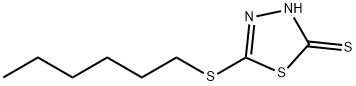
2-N-HEXYLTHIO-1,3,4-THIADIAZOLE-5-THIOL synthesis
- Product Name:2-N-HEXYLTHIO-1,3,4-THIADIAZOLE-5-THIOL
- CAS Number:4858-28-0
- Molecular formula:C8H14N2S3
- Molecular Weight:234.41

111-25-1
322 suppliers
$10.00/5g
1072-71-5
388 suppliers
$10.00/1g

4858-28-0
25 suppliers
$112.00/1g
Yield:-
Reaction Conditions:
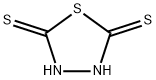
Stage #1: 2,5-Dimercapto-1,3,4-thiadiazolewith potassium hydroxide in ethanol at 75; for 2 h;Inert atmosphere;
Stage #2: 1-bromo-hexane in ethanol at 77; for 6 h;Inert atmosphere;
Steps:
24 Example 24. Synthesis of 2-(n-Hexyl)thio-5-thiol-1,3,4-Thiadiazole
150.2 grams of DMTD and 900 mL of ethanol were added to a 3-L flask and 56.1 grams of potassium hydroxide was added in portions over a 30 minute period. The reaction was heated at 75 C for 2 hours under nitrogen and cooled to 24 C. 165.1 grams of n-hex- ylbromide was added over 20 minutes and the mixture heated at reflux (77 C) for 6 hours. Ethanol was distilled from the reaction and the residue dissolved into 400 grams of wa- ter/600 mL of toluene and transferred to a separatory funnel. The organic phase was washed with additional water, collected, and dried over anhydrous sodium sulfate. After removal of the toluene on a rotary evaporator, the low-melting solid obtained was recrys- tallized from reagent grade toluene:n-hexane (80:20) wt.:wt. and dried in a vacuum oven to obtain the pure product.
References:
WO2021/55388,2021,A1 Location in patent:Paragraph 0110