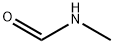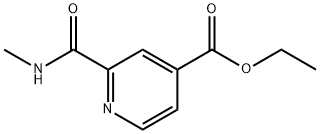
2-METHYLCARBAMOYLISONICOTINIC ACID ETHYL ESTER synthesis
- Product Name:2-METHYLCARBAMOYLISONICOTINIC ACID ETHYL ESTER
- CAS Number:332013-42-0
- Molecular formula:C10H12N2O3
- Molecular Weight:208.21
Yield:332013-42-0 79.2%
Reaction Conditions:
with sulfuric acid;dihydrogen peroxide;iron(II) sulfate in water at 6 - 22; for 0.5 h;
Steps:
H.1
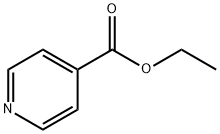
A stirred solution of ethyl isonicotinate (250 mL, 1.64 mole) and concentrated sulfuric acid (92 mL, 1.64 mole) in N-methylformamide (2.0 L) was cooled to 6°C with an ice bath. Iron (II) sulfate heptahydrate (22.8 g, 0.0812 mole, milled with a mortar and pestle) was added, followed by the dropwise addition of 30% aqueous hydrogen peroxide (56 mL, 0.492 mole). The additions of iron (II) sulfate and hydrogen peroxide were repeated four additional times, while the reaction temperature was kept below 22°C. After the reaction mixture was stirred for thirty minutes, sodium citrate solution (2 L, 1 M) was added (pH of the resulting mixture was about 5). The mixture was extracted with dichloromethane (1L, 2 x 500 mL). The combined organic extracts were washed with water (2 x 500 mL), 5% aqueous sodium bicarbonate (3 x 100 mL), and brine (500 mL). The resulting organic solution was then dried over sodium sulfate, filtered and concentrated in vacuo to afford a solid. The crude solid was triturated with hexanes, filtered, washed with hexanes and dried under vacuum to give 270.35 g (79.2%) of pastel yellow solid. 1H NMR (DMSO-d6, 300 MHz): δ 8.9 (d, 1H), 8.3 (m, 1H), 8.0 (dd, 1H), 4.4 (q, 2H), 2.8 (d, 3H), 1.3 (t, 3H).
References:
EP1228063,2009,B1 Location in patent:Page/Page column 41