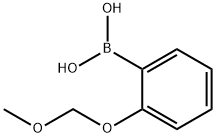
2-(METHOXYMETHOXY)PHENYLBORONIC ACID synthesis
- Product Name:2-(METHOXYMETHOXY)PHENYLBORONIC ACID
- CAS Number:115377-93-0
- Molecular formula:C8H11BO4
- Molecular Weight:181.98
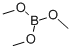
121-43-7
356 suppliers
$13.00/25mL
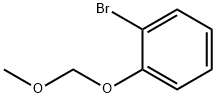
68314-54-5
12 suppliers
$49.00/200 μmol
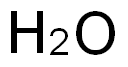
7732-18-5
471 suppliers
$12.69/100ml

115377-93-0
57 suppliers
$31.00/1g
Yield: 71%
Reaction Conditions:
Stage #1:1-bromo-2-(methoxymethoxy)benzene with tert.-butyl lithium in tetrahydrofuran;pentane at -78; for 1 h;Inert atmosphere;
Stage #2:Trimethyl borate in tetrahydrofuran;pentane at -78 - 20; for 2 h;Inert atmosphere;
Stage #3:water with ammonium chloride in tetrahydrofuran;pentaneInert atmosphere;
Steps:
2.1.1 2-(Methoxymethoxy)phenylboronic acid (8a)
Under an argon atmosphere, apentane solution of tert-butyllithium (1.52 M, 14.4 mL, 21.9 mmol) was added dropwise to a solution of S1 (2.38 g, 11.0 mmol) in THF (30 mL) at -78 °C. After stirring for 1 h at -78 °C, trimethyl borate (1.84 mL, 16.5 mmol) was added as a neat liquid and the mixture was stirred for 1 h at -78 °C. The reaction mixture was allowed to warmto room temperature and stirred for an additional 1 h. The mixture was quenched with saturatedaqueous NH4Cl and concentrated. The products were adjusted to pH 3 with acetic acid and themixture was extract with dichloromethane. The extract was washed successively with water and brine, dried over Na2SO4, and evaporated. The residue was washed with hexane and dried underreduced pressure to give 8a as a colorless powder (1.41 g, 71%). This compound was used for thenext reaction without further purification.
References:
Fukuda, Tsutomu;Umeki, Teppei;Tokushima, Keiji;Xiang, Gao;Yoshida, Yuki;Ishibashi, Fumito;Oku, Yusuke;Nishiya, Naoyuki;Uehara, Yoshimasa;Iwao, Masatomo [Bioorganic and Medicinal Chemistry,2017,vol. 25,# 24,p. 6563 - 6580] Location in patent:supporting information
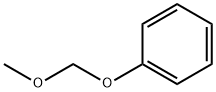
824-91-9
19 suppliers
inquiry

115377-93-0
57 suppliers
$31.00/1g

68314-54-5
12 suppliers
$49.00/200 μmol

115377-93-0
57 suppliers
$31.00/1g
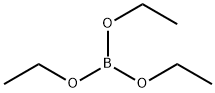
150-46-9
197 suppliers
$12.00/25mL

68314-54-5
12 suppliers
$49.00/200 μmol
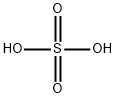
7664-93-9
6 suppliers
$21.64/1003

115377-93-0
57 suppliers
$31.00/1g
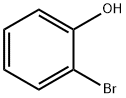
95-56-7
299 suppliers
$9.00/5 g

115377-93-0
57 suppliers
$31.00/1g