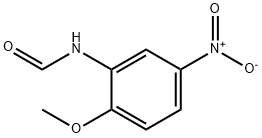
2-METHOXY-5-NITROFORMANILIDE synthesis
- Product Name:2-METHOXY-5-NITROFORMANILIDE
- CAS Number:149686-06-6
- Molecular formula:C8H8N2O4
- Molecular Weight:196.16
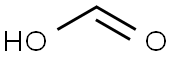
64-18-6
1119 suppliers
$22.12/250ML
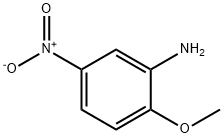
99-59-2
211 suppliers
$15.00/5mg

149686-06-6
17 suppliers
$28.60/250mg
Yield:149686-06-6 99.1%
Reaction Conditions:
Stage #1: formic acidwith acetic anhydride at 0 - 32; for 1 h;
Stage #2: 3-amino-4-methoxynitrobenzene at 32;
Steps:
1.B; a
Into a 5-L, 3 -neck flask equipped with a mechanical stirrer, a temperature probe and an ice bath was placed, acetic anhydride (367 g, 9.72 mol). Fo?nic acid (367 g, 6.01 mol) was added with stirring and cooling. The rate of addition was such that the temperature did not exceed 32 0C. Stirring was continued for one hour at ambient temperature. Cooling was again applied and 2-methoxy-5-nitroaniline (3) (415 g, 2.47 mol) was added in portions over a 1.5-hour period. The temperature was kept below 32 0C during addition. The thick-yellow slurry was stirred overnight.Water (3 L) was added and the mixture was stirred for an additional 48 hours. The yellow product was collected by filtration and washed with water until the pH of the aqueous washing was neutral. A total of 9.2 L of water was used. The product was dried at 60 0C under house vacuum and afforded 480 g (99.1 %) of the title compound.; As shown in Figure 1, the linear PQQ synthesis began with the addition of commercially available (Aldrich, Milwaukee, WI) 2-methoxy-5-nitroaniline (3) to a cooled solution of acetic anhydride (Ac2O) and formic acid to afford a thick slurry, which was stirred overnight. Water was added to the reaction mixture which was then stirred for an additional two days. The pale product was collected by vacuum filtration and washed with water until the pH of the washing solution was neutral. This first step of the synthesis afforded after drying on the house vacuum, N-(2-methoxy-5-nitrophenyl)formamide (4).
References:
WO2006/102642,2006,A1 Location in patent:Page/Page column 8; 13; 1/6
108-05-4
590 suppliers
$4.00/1000g

99-59-2
211 suppliers
$15.00/5mg

149686-06-6
17 suppliers
$28.60/250mg