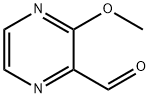
2-FORMYL-3-METHOXYPYRAZINE synthesis
- Product Name:2-FORMYL-3-METHOXYPYRAZINE
- CAS Number:63874-90-8
- Molecular formula:C6H6N2O2
- Molecular Weight:138.12
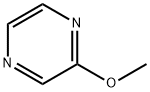
3149-28-8
253 suppliers
$7.00/5g
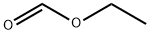
109-94-4
477 suppliers
$10.00/10g

63874-90-8
31 suppliers
$220.00/50mg
Yield:63874-90-8 70%
Reaction Conditions:
Stage #1: 2-methoxypyrazinewith 2,2,6,6-tetramethylpiperidinyl-lithium in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Stage #2: formic acid ethyl ester in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Steps:
2 4.2. 3-Methoxypyrazine-2-carbaldehyde (11)
To a solution of lithium 2,2,6,6-tetra-methylpiperidide (2.6 mmol, 0.5 M in THF) at -78 °C was added a solution of 2-methoxypyrazine (220 mg, 2.0 mmol) in THF (5 mL). The reaction mixture was stirred at -78 °C for 30 min. Ethyl formate (167 mg, 0.18 mL, 2.3 mmol) was added and stirring was continued for 30 min at -78 °C. A mixture of hydrochloric acid (35% aq, 1 mL), ethanol (1 mL) and THF (4 mL) was added and the resulting mixture was warmed to room temperature, neutralized with NaHCO3 (satd, 5 mL) and evaporated to near dryness. The residue was extracted with dichloromethane (3×25 mL). The organic extract is dried (MgSO4) and reduced in vacuo. The crude residue was purified by flash column chromatography using n-hexanes/ethyl acetate (3:1) as eluent to give the title compound as a yellow solid (193 mg, 1.4 mmol, 70%); mp 47-49 °C (lit.10 mp 66-67 °C); νmax (neat)/cm-1 2955, 2797, 1710, 1534, 1468, 1365, 1305, 1072, 954, 762; δH (400 MHz, CDCl3) 10.27 (1H, s, CHO), 8.37 (1H, d, J 2.4, CH), 8.35 (1H, d, J 2.4, CH), 4.13 (3H, s, Me); δC (100 MHz, CDCl3) 190.1 (CHO), 160.5 (C), 145.7 (CH), 137.5 (CH), 136.3 (C), 54.5 (Me); m/z (ESI) 161 (100%, [M+Na]+); HRMS (ESI, [M+Na]+) found 161.0322. [C6H6N2NaO2]+ requires 161.0321.
References:
Wang, Christy;Sperry, Jonathan [Tetrahedron,2014,vol. 70,# 21,p. 3430 - 3439]