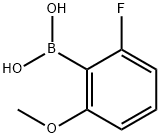
2-Fluoro-6-methoxyphenylboronic acid synthesis
- Product Name:2-Fluoro-6-methoxyphenylboronic acid
- CAS Number:78495-63-3
- Molecular formula:C7H8BFO3
- Molecular Weight:169.95
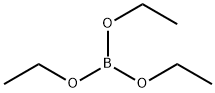
150-46-9
197 suppliers
$12.00/25mL
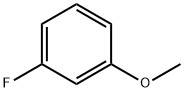
456-49-5
360 suppliers
$8.00/10g

78495-63-3
246 suppliers
$9.00/1g
Yield:-
Reaction Conditions:
Stage #1:1-fluoro-3-methoxybenzene with n-butyllithium;N-ethyl-N,N-diisopropylamine in tetrahydrofuran at -70; for 0.0833333 h;
Stage #2:triethyl borate in tetrahydrofuran at -70; for 0.166667 h;
Steps:
A1 Step A1 Reaction Scheme and Charge Table
Reactor A was charged with THF (6 vol) and Diisopropylamine (1.4 equiv). The resulting solution was cooled to -70 °C and n-BuLi (2.5 M in hexane, 1.5 equiv) was slowly added. After addition is complete, a solution of 3-fluoroanisole (1.0 equiv) in THF (6 vol) was added slowly and kept at -70 °C for 5 min. B(EtO)3 (2.0 equiv) was added slowly and kept at -70 °C for 10 min. The reaction mixture was quenched with 2N HC1. The quenched reaction mixture was extracted with MTBE (3 x 4 vol). The combined organic phases were concentrated to 1.5-3 total volumes. Heptane (7-9 vol) was added drop-wise and the mixture was cooled to 0-10 °C and stirred for 3 h. The mixture was filtrated and rinsed with heptane (1.5 vol). The solid was dried under nitrogen at < 30 °C to afford (2-fluoro-6-methoxyphenyl)boronic acid.
References:
AMGEN INC.;PARSONS, Andrew Thomas;COCHRAN, Brian McNeil;POWAZINIK, IV, William;CAPORINI, Marc Anthony WO2020/102730, 2020, A1 Location in patent:Page/Page column 70-71