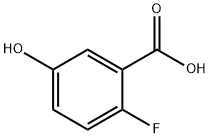
2-FLUORO-5-HYDROXYBENZOIC ACID synthesis
- Product Name:2-FLUORO-5-HYDROXYBENZOIC ACID
- CAS Number:51446-30-1
- Molecular formula:C7H5FO3
- Molecular Weight:156.11
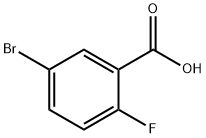
146328-85-0
280 suppliers
$6.00/1g

51446-30-1
156 suppliers
$18.00/1g
Yield: 87%
Reaction Conditions:
Stage #1:5-bromo-2-fluoro-benzoic acid with water;sodium carbonate for 1 h;Reflux;
Stage #2: with (R,R)-N,N'-dimethyl-1,2-diaminocyclohexane;copper(ll) bromideReflux;
Steps:
n
Na2CO3 (1 eq.) was added to a degassed souUon of water (0.35 M) and the appropriatebromobenzoc acid (1 eq.) and was refluxed for 30 minutes. A further portion of Na2CO3 (1.5eq.) was added to the reaction and refluxed for a further 30 mnutes. Separat&y CuBr2 (0.1 eq.) and trans-N, W-dmethycycohexane-1 ,2-damne (0.2 eq.) were added to degassed water (0.04 M) and an ntense bue coour was observed. This mixture was added to the refluxng aqueous souton and aflowed to sUr at this temperature overnight. The resuWngsouton was aflowed to coo to room temperature and was acid Wed with conc. HC and extracted nto EtOAc. The organcs were dried with MgSO4, ffltered, and the sovent removed in vacuo to give the desired product.The foHowng compounds were made by the Uflmann transformaUon method A Al)H5bromo-2- fluorobenzoc acid (3.5g, 15.98 mmo)Off whte sohd (2.16g, 87% yed). 1H NMR(400 MHz, DM80) 6 7.18 (dd, J = 6.0, 3.2Hz, 1H), 7,09 (dd, J= 10.5, 8.9 Hz, 1H), 6.98 6.93 (m, 1H). LCM8 B rt 3.19 mn, m/z157.1 [M + H].
References:
MONASH UNIVERSITY;THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH;VOSS, Anne Kathrin;BAELL, Jonathan;NGUYEN, Huu Nghi;LEAVER, David J.;CLEARY, Benjamin L.;LAGIAKOS, H. Rachel;SHEIKH, Bilal Nadeem;THOMAS, Timothy John WO2016/198507, 2016, A1 Location in patent:Page/Page column 40; 47
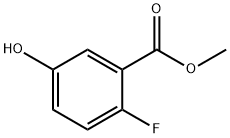
1084801-91-1
67 suppliers
$30.00/1g

51446-30-1
156 suppliers
$18.00/1g
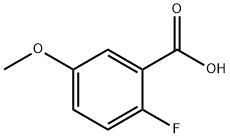
367-83-9
116 suppliers
$20.00/1 g

51446-30-1
156 suppliers
$18.00/1g
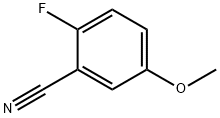
127667-01-0
183 suppliers
$10.00/1g

51446-30-1
156 suppliers
$18.00/1g
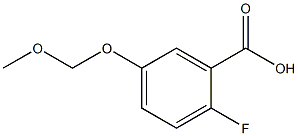
368422-25-7
1 suppliers
inquiry

51446-30-1
156 suppliers
$18.00/1g