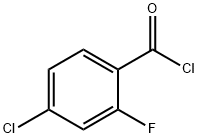
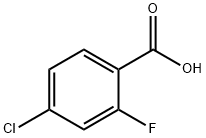
2-Fluoro-4-chlorobenzoyl chloride synthesis
- Product Name:2-Fluoro-4-chlorobenzoyl chloride
- CAS Number:394-39-8
- Molecular formula:C7H3Cl2FO
- Molecular Weight:193
446-30-0
294 suppliers
$7.00/5g

394-39-8
130 suppliers
$12.00/1g
Yield:394-39-8 100%
Reaction Conditions:
with oxalyl dichloride in tetrahydrofuran;DMF (N,N-dimethyl-formamide) at 38 - 42;
Steps:
1
Example 1.; Preparation of the benzoyl chloride: F O HO CI CI oe tCI CIXCI p /O 2-fluoro-4-2-fluoro-4chlorobenzoyl chlorobenzoic acid oxalyl chloride chloride (compound 17) (compound 18) (compound 6) [0085] A 12L round bottom flask was equipped with a large diameter gas outlet tube, 1 L addition funnel, nitrogen sweep, and overhead stirrer. To this vessel was charged 1360g (7.79 moles, 1 equivalent) of 2-fluoro-4-chlorobenzoic acid. This was followed by addition of 5.0 liters of dry tetrahydrofuran (THF), which readily dissolved the white fluffy solid to give a yellowish clear solution. To this stirring solution was added 13.6 g of dimethylformamide (DMF). Oxalyl chloride (1088g, 8. 57 moles, 1.1 equivalent) placed in the addition funnel was added dropwise. As the addition progresses, the batch temperature increased to ca. 38°C. Afterwards the batch was heated to 42°C and held until no remaining starting material was left. The batch was cooled to room temperature and a nitrogen sweep was started to remove HC1 and excess oxalyl chloride along with tetrahydrofuran. The reactor was then put under a vacuum to remove tetrahydrofuran and isolate the benzoyl chloride product as a pale yellow oil. Final residual solvent was removed under pump vacuum and product was filtered under nitrogen through a coarse fritted glass filter. Near quantitative yield of the benzoyl chloride obtained in this manner can be utilized in the subsequent chemistry without further purification. Samples will crystallize in large crystals if left in the refrigerator but will re-melt at 25 °C. [0086] GC retention time of the benzoyl chloride was 7. 17min. Column : 30M DB-5 cap column, He 18psig ; 50°C, hold 2min., 20°C/minute to 250°C.'H NMR (CDC13) 6 8.07 (m, l H), 7.25 (m, 2H).
References:
PHARMACIA CORPORATION WO2005/61486, 2005, A1 Location in patent:Page/Page column 21-22
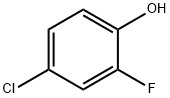
348-62-9
212 suppliers
$7.00/5g

394-39-8
130 suppliers
$12.00/1g
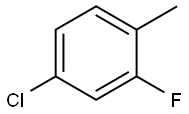
452-75-5
254 suppliers
$16.00/25 g

394-39-8
130 suppliers
$12.00/1g