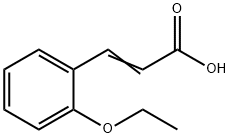
2-ETHOXYCINNAMIC ACID synthesis
- Product Name:2-ETHOXYCINNAMIC ACID
- CAS Number:69038-81-9
- Molecular formula:C11H12O3
- Molecular Weight:192.21
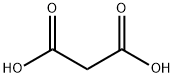
141-82-2
752 suppliers
$5.00/25g
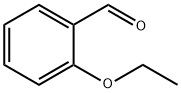
613-69-4
253 suppliers
$11.00/5g

69038-81-9
68 suppliers
$21.21/500mgs:
Yield:-
Reaction Conditions:
with piperidine in pyridine at 80; for 6 h;
Steps:
General procedure for the preparation of furoxan oxynitride 9a-9g
General procedure: Step 1: To a solution of aryl formaldehyde (6a~6g, 20 mmol) and malonic acid in pyridine (40 mL) was added piperidine (2.55 g, 30 mmol). The resulting mixture was heated to 80 °C for 6 hours until no starting material was detected by TLC. Then the solvent was removed in vacuum to give viscous liquid. The crude product was diluted with 1 M aqueous NaOH solution (200 mL) and was washed with EtOAc (100 mL × 3). The aqueous layer was acidified to pH=1 with HCl (aq., 2M), and extracted with EtOAc (150 mL × 3). The combined organic layer was washed with brine, dried over anhydrous sodium sulfate, and concentrated in vacuum to afford aryl acrylic acid as white solid in 60-78% yields. Step 2: To a solution of aryl acrylic acid 7a~7j (10 mmol) in anhydrous THF under argon at 0 °C was added a solution of BH3 (1.0 M in THF, 15 mL) drop wise. The resulting solution was stirred at room temperature until no starting material was detected by TLC. The reaction was quenched with H2O (100 mL) and extracted with EtOAc (80 mL × 3). The combined organic layer was washed with brine, dried over anhydrous sodium sulfate, and concentrated in vacuum to give the crude product, which was purified by silica gel column chromatography (PE : EA = 4 : 1) to obtain the aryl propylene alcohol 8a~8g as light yellow oil in 67 % to 92 % yields. Step 3: To a solution of aryl propylene alcohol 8a~8g (5 mmol) in AcOH (1 mL) at 0 °C under argon was added a saturated aqueous NaNO2 (1.04 g, 15 mmol) solution drop wise over 30 min. The mixture was stirred at room temperature overnight. The reaction was quenched with H2O (30 mL) and extracted with EtOAc (50 mL × 3). The combined organic layer was washed with saturated Na2CO3 and brine, dried with anhydrous Na2SO4, and concentrated in vacuum. The crude product was purified by silica gel column chromatography (PE:EA = 10 : 1 to 5 : 1) to afford 3-(hydroxymethyl)-4-aryl-furoxan as white or yellow solid. Step 4: The product in step 3 (1 mmol) was dissolved in anhydrous DCM at 0 °C. Then TEA (151 mg, 1.5 mmol) and MsCl (125 mg, 1.1 mmol) were added successively. The resulting mixture was stirred at room temperature until no starting material was detected by TLC, The solvent was then removed in vacuum and the residue was purified by silica gel column chromatography (PE : EA = 20 : 1) to give the furoxan derivatives 9a~9g in 29 % to 46 % yield for 2 steps.
References:
Ma, Jinjin;Chen, Lan;Fan, Jinbao;Cao, Wei;Zeng, Guangyao;Wang, Yajing;Li, Yuanjian;Zhou, Yingjun;Deng, Xu [European Journal of Medicinal Chemistry,2019,vol. 168,p. 146 - 153] Location in patent:supporting information

141-82-2
752 suppliers
$5.00/25g

613-69-4
253 suppliers
$11.00/5g
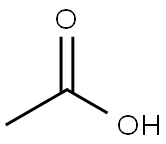
64-19-7
1559 suppliers
$10.00/25ML

69038-81-9
68 suppliers
$21.21/500mgs:
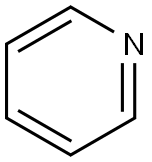
110-86-1
852 suppliers
$9.00/5g

141-82-2
752 suppliers
$5.00/25g

613-69-4
253 suppliers
$11.00/5g

69038-81-9
68 suppliers
$21.21/500mgs: