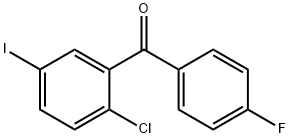
(2-Chloro-5-iodophenyl)(4-fluorophenyl)methanone synthesis
- Product Name:(2-Chloro-5-iodophenyl)(4-fluorophenyl)methanone
- CAS Number:915095-86-2
- Molecular formula:C13H7ClFIO
- Molecular Weight:360.55
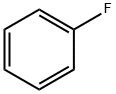
462-06-6
431 suppliers
$10.00/5g
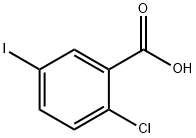
19094-56-5
541 suppliers
$6.00/5g

915095-86-2
235 suppliers
$5.00/250mg
Yield:915095-86-2 94%
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide at 25 - 30; for 5 h;
Steps:
1
Example 1 : Synthesis of the fluoride VIII.1Oxalylchloride (176kg; 1386mol; 1 ,14eq) is added to a mixture of 2-chloro-5-iodo benzoic acid (343kg; 1214mol) (compound IX.1 ), fluorobenzene (858kg) and N,N- dimethylformamide (2kg) within 3 hours at a temperature in the range from about 25 to 30°C (gas formation). After completion of the addition, the reaction mixture is stirred for additional 2 hours at a temperature of about 25 to 30°C. The solvent (291 kg) is distilled off at a temperature between 40 and 45°C (p=200mbar). Then the reaction solution (91 1 kg) is added to aluminiumchloride AICI3 (181 kg) andfluorobenzene (192kg) at a temperature between about 25 and 30°C within 2 hours. The reaction solution is stirred at the same temperature for about an additional hour. Then the reaction mixture is added to an amount of 570 kg of water within about 2 hours at a temperature between about 20 and 30°C and stirred for an additional hour. After phase separation the organic phase (1200kg) is separated into two halves (600kg each). From the first half of the organic phase solvent (172kg) is distilled off at a temperature of about 40 to 50°C (p=200mbar). Then 2-propanole (640kg) is added. The solution is heated to about 50°C and then filtered through a charcoal cartouche (clear filtration). The cartouche may be exchanged during filtration and washed with a fluorobenzene/2-propanole mixture (1 :4; 40kg) after filtration. Solvent (721 kg) is distilled off at a temperature of about 40 to 50°C and p=200mbar. Then 2-propanole (240kg) is added at a temperature in the range between about 40 to 50°C. If the content of fluorobenzene is greater than 1 % as determined via GC, another 140kg of solvent are distilled off and 2-propanole (140kg) is added. Then the solution is cooled from about 50°C to 40°C within one hour and seeding crystals (50g) are added. The solution is further cooled from about 40°C to 20°C within 2 hours. Water (450kg) is added at about 20°C within 1 hour and the suspension is stirred at about 20°C for an additional hour before the suspension is filtered. The filter cake is washed with 2-propanole/water (1 :1 ; 800kg). The product is dried until a water level of <0.06%w/w is obtained. The second half of the organic phase is processed identically. A total of 410kg (94%yield) of product which has a white to off-white crystalline appearance, is obtained. The identity of the product is determined via infrared spectrometry.
References:
WO2011/39107,2011,A1 Location in patent:Page/Page column 18-19
281652-58-2
25 suppliers
inquiry

462-06-6
431 suppliers
$10.00/5g

915095-86-2
235 suppliers
$5.00/250mg

19094-56-5
541 suppliers
$6.00/5g

915095-86-2
235 suppliers
$5.00/250mg
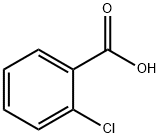
118-91-2
676 suppliers
$14.00/25g

915095-86-2
235 suppliers
$5.00/250mg
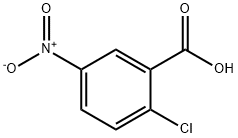
2516-96-3
461 suppliers
$6.00/25g

915095-86-2
235 suppliers
$5.00/250mg