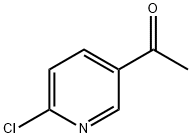
2-Chloro-5-acetylpyridine synthesis
- Product Name:2-Chloro-5-acetylpyridine
- CAS Number:55676-22-7
- Molecular formula:C7H6ClNO
- Molecular Weight:155.58
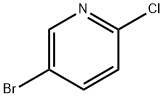
53939-30-3
460 suppliers
$5.00/1g
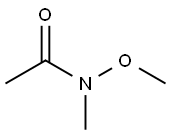
78191-00-1
280 suppliers
$6.00/5g

55676-22-7
270 suppliers
$7.00/100mg
Yield:55676-22-7 84%
Reaction Conditions:
Stage #1: 5-bromo-2-chloropyridinewith TurboGrignard in tetrahydrofuran at 0; for 0.25 h;Inert atmosphere;
Stage #2: N-Methoxy-N-methylacetamide in tetrahydrofuran at 0 - 20;Inert atmosphere;
Steps:
IM15: 1-(6-Chloro-pyridin-3-yl)-ethanone
A round bottomed flask was charged with 5-bromo-2-chloropyridine (5.30 g, 27.6 mmol) in THF under N2 and cooled at 0° C. A solution of 1 M iso-propylmagnesiumchloride-lithium chloride complex in THF (40 mL) was added drop wise over 15 min. After 70 min N-methoxy-N-methylacetamide (4.1 mL, 38 mmol) was added drop wise. After stirring for 5 min at 0° C. the cooling bath was removed. The mixture was left stirring overnight and was then quenched by the addition of 100 mL saturated aqueous NH4Cl solution. The mixture was extracted with 3×100 mL EtOAc. The combined organic layers were washed with water followed by brine and dried over MgSO4. Evaporation of the volatiles at 80° C., 10 mbar for 1 h gave the title compound (3.596 g, 84) sufficiently pure for the next step.
References:
US2013/12530,2013,A1 Location in patent:Paragraph 0187
923034-23-5
10 suppliers
inquiry

55676-22-7
270 suppliers
$7.00/100mg
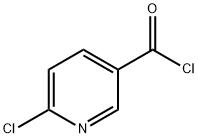
58757-38-3
204 suppliers
$6.00/1g
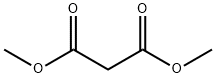
108-59-8
602 suppliers
$5.00/25g

55676-22-7
270 suppliers
$7.00/100mg

58757-38-3
204 suppliers
$6.00/1g

55676-22-7
270 suppliers
$7.00/100mg
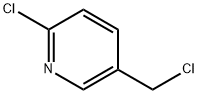
70258-18-3
515 suppliers
$5.00/5g

108-59-8
602 suppliers
$5.00/25g

55676-22-7
270 suppliers
$7.00/100mg