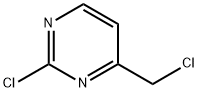
2-Chloro-4-(chloromethyl)pyrimidine synthesis
- Product Name:2-Chloro-4-(chloromethyl)pyrimidine
- CAS Number:944902-31-2
- Molecular formula:C5H4Cl2N2
- Molecular Weight:163
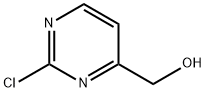
34953-87-2
52 suppliers
inquiry

944902-31-2
60 suppliers
$45.00/10mg
Yield: 74%
Reaction Conditions:
with thionyl chloride in dichloromethane at 20;
Steps:
135
To a stirred solution of (2-chloropyrimidin-4-yl)methanol (0.600 g, 4.14 mmol) in dichloromethane (10 mL) was added thionyl chloride (0.488 g, 4.14 mmol). The mixture was stirred at room temperature overnight and then concentrated to afford 2-chloro-4- (chloromethyl)pyrimidine as a yellow oil (0.500 g, 74%>). To a stirred solution of this intermediate (0.500 g, 3.07 mmol) and 4-fiuorophenol (0.378 g, 3.37 mmol) in acetonitrile (10 mL) was added potassium carbonate (0.847 g, 6.14 mmol). The reaction was heated at reflux for 1.5 hours and then concentrated. The residue was purified by flash chromatography over silica gel using a hexane/ethyl acetate eluant to afford 2- chloro-4-((4-fluorophenoxy)methyl)pyrimidine as a white solid (0.300 g, 41%). To a stirred solution of this compound (0.490 g, 2.06 mmol) in acetonitrile (15 mL) was added ethyl 4-fluoropiperidine-4-carboxylate hydrochloride (0.480 g, 2.26 mmol) and potassium carbonate (0.569 g, 4.12 mmol). The reaction was heated at reflux overnight, diluted with water (20 mL) and extracted with ethyl acetate. The combined organic layers were washed with brine, dried (Na2S04) and concentrated. The residue was purified by flash chromatography over silica gel using a hexane/ethyl acetate eluant to afford ethyl 4- fiuoro-l-(4-((4-fluorophenoxy)methyl)pyrimidin-2-yl)piperidine-4-carboxylate as a white solid (0.400 g, 53%). Exchanging ethyl l-(4-(4-fluorophenyl)pyrimidin-2-yl)piperidine-4- carboxylate for the present intermediate, the final two steps of Example 41 were used to prepare the title compound. 1H NMR (400 MHz, CDC13) δ 8.30 (d, J = 4.8 Hz, 1H), 7.01- 6.97 (m, 2H), 6.90-6.87 (m, 2H), 6.69 (d, J = 4.8 Hz, 1H), 6.38 (d, J = 6.8 Hz, 1H), 4.93 (s, 2H), 4.75 (d, J = 12.8 Hz, 2H), 3.26-3.20 (m, 2H), 3.00-2.80 (m, 6H), 2.32-2.12 (m, 3H), 1.88-1.77 (m, 4H ), 1.59-1.50 (m, 5H) ppm. 13C NMR (100 MHz, CDC13) δ 171.3, 171.1, 166.7, 161.1, 158.6, 158.4, 156.3, 154.3, 154.3, 116.0, 115.8, 115.7, 115.6, 106.5, 96.9, 95.1, 70.3, 63.0, 52.8, 46.5, 46.3, 39.1, 32.0, 31.9, 31.8, 31.7, 30.1, 24.2, 22.9, 22.3 ppm. Purity: > 95% LCMS (214 nm & 254 nm); retention time 1.42 min; (M+H+) 472.2.
References:
Location in patent:Page/Page column 224; 225
13036-57-2
334 suppliers
$6.00/1g

944902-31-2
60 suppliers
$45.00/10mg