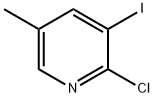
2-CHLORO-3-IODO-5-PICOLINE synthesis
- Product Name:2-CHLORO-3-IODO-5-PICOLINE
- CAS Number:59782-91-1
- Molecular formula:C6H5ClIN
- Molecular Weight:253.47
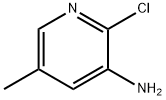
34552-13-1
218 suppliers
$9.00/1g

59782-91-1
70 suppliers
$65.00/50 mg
Yield: 93%
Reaction Conditions:
Stage #1:3-Amino-2-chloro-5-methylpyridine with hydrogenchloride;sodium nitrite in water at -15 - -10; for 0.25 h;Sandmeyer Reaction;
Stage #2: with potassium iodide in water at -5 - 20; for 3.5 h;Sandmeyer Reaction;
Steps:
2-Chloro-3-iodo-5-methylpyridine (20).
From compound 22: To a solution of 2-chloro-3-aminopicoline (22) (500 mg, 3.51 mmol) in a concentrated aqueous hydrochloric acid solution (37%, w/w, 4 mL), was added dropwise at -15 °C, a solution of sodium nitrite (363 mg, 5.26 mmol) in water (10 mL) while maintaining the temperature below -10 °C. The reaction was stirred at -10 °C for 15 min, then a solution of potassium iodide (1.75 g, 10.52 mmol) in water (10 mL) was added dropwise while maintaining the temperature below -5 °C. The reaction was stirred at 0 °C for 30 min then at room temperature for 3 h. The reaction was quenched by a slow addition of a 10 N aqueous sodium hydroxide solution until pH = 11. After extraction with dichloromethane (3 x 30 mL), the combined organic layers were dried over magnesium sulfate, filtered and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel (ethyl acetate/cyclohexane, 2/8, v/v) to give 2-chloro-3-iodo-5-methylpyridine (20) (827 mg, 3.26 mmol) as a yellow solid. Yield 93%. Rf (ethyl acetate/cyclohexane, 2/8, v/v) 0.28; mp 58-60 °C (litt.[3]: 59-61 °C); IR (KBr) ν 1402, 1459, 2921 cm-1; 1H NMR (200 MHz, CDCl3) δ 2.26 (s, 3H, CH3), 7.96 (d, 1H, 2.0 Hz, H-4), 8.15 (d, 1H, J = 2.0 Hz, H-6); 13C NMR (50 MHz, CDCl3) δ 17.3 (CH3), 94.4 (C-3), 133.6 (C-5), 149.2 (C-6), 149.4 (C-4), 151.7 (C-2); ESI-MS m/z 253.89 [M+H]+.
References:
Billaud, Emilie M.F.;Maisonial-Besset, Aurélie;Rbah-Vidal, Latifa;Vidal, Aurélien;Besse, Sophie;Béquignat, Jean-Baptiste;Decombat, Caroline;Degoul, Fran?oise;Audin, Laurent;Deloye, Jean-Bernard;Dollé, Frédéric;Kuhnast, Bertrand;Madelmont, Jean-Claude;Tarrit, Sébastien;Galmier, Marie-Josèphe;Borel, Michèle;Auzeloux, Philippe;Miot-Noirault, Elisabeth;Chezal, Jean-Michel [European Journal of Medicinal Chemistry,2015,vol. 92,p. 818 - 838] Location in patent:supporting information
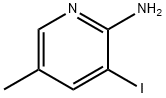
211308-79-1
75 suppliers
$28.00/250mg

59782-91-1
70 suppliers
$65.00/50 mg
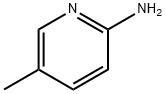
1603-41-4
540 suppliers
$7.00/25g

59782-91-1
70 suppliers
$65.00/50 mg