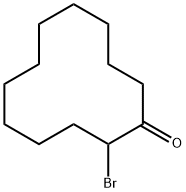
2-bromocyclododecanone synthesis
- Product Name:2-bromocyclododecanone
- CAS Number:31236-94-9
- Molecular formula:C12H21BrO
- Molecular Weight:261.2
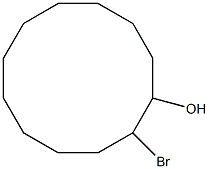
83602-77-1
0 suppliers
inquiry

31236-94-9
7 suppliers
$28.60/250mg
Yield:31236-94-9 126 mg
Reaction Conditions:
with oxone;2-iodo-3,4,5,6-tetramethylbenzoic acid in water;acetonitrile at 25 - 30; for 24 h;
Steps:
4.1 General procedures for α-bromoketones and α-azidoketones from olefins
General procedure: To a solution of the olefin (0.5-1.2mmol) in 10-16mL of acetonitrile-water (1:1) mixture was added NBS (1.05equiv) at rt. The reaction mixture was stirred until all the olefin was consumed, as monitored by TLC analysis. Subsequently, TetMe-IA (10mol%) and Oxone (1.0equiv) were introduced into the reaction mixture, and the stirring was continued. After completion of the oxidation as judged by TLC analysis, the reaction mixture was washed with saturated NaHCO3 solution. The organic matter was extracted 2-3 times with ethyl acetate or dichloromethane, and the combined organic extract was dried over anhyd Na2SO4. The solvent was removed in vacuo and the resultant residue was subjected to a short-pad silica gel column chromatography to isolate pure α-bromoketone.
References:
Chandra, Ajeet;Parida, Keshaba Nanda;Moorthy, Jarugu Narasimha [Tetrahedron,2017,vol. 73,# 40,p. 5827 - 5832]
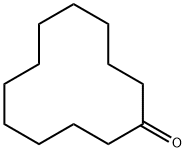
830-13-7
156 suppliers
$22.00/25g

31236-94-9
7 suppliers
$28.60/250mg
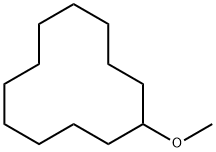
2986-54-1
23 suppliers
inquiry

31236-94-9
7 suppliers
$28.60/250mg
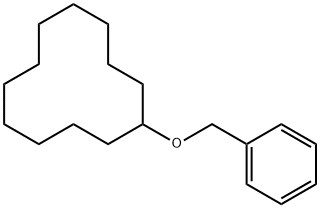
121758-10-9
0 suppliers
inquiry

31236-94-9
7 suppliers
$28.60/250mg
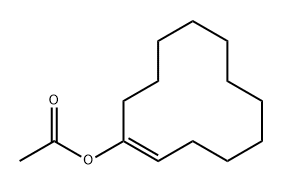
71365-98-5
0 suppliers
inquiry

31236-94-9
7 suppliers
$28.60/250mg