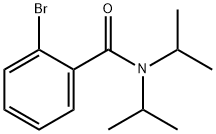
2-Bromo-N,N-diisopropylbenzamide synthesis
- Product Name:2-Bromo-N,N-diisopropylbenzamide
- CAS Number:79839-66-0
- Molecular formula:C13H18BrNO
- Molecular Weight:284.19
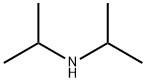
7154-66-7
167 suppliers
$10.00/5g
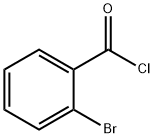
108-18-9
364 suppliers
$10.00/1g

79839-66-0
19 suppliers
$45.00/100mg
Yield:-
Reaction Conditions:
in dichloromethane at 5 - 20;
Steps:
1
SYNTHESIS EXAMPLE 1; (Synthesis of Compound 1a [see formula (20) given later]); Into a 300-mL eggplant type flask were introduced 46.0 g (455 mmol) of diisopropylamine and 150 mL of dichloromethane. This liquid reaction mixture was cooled to 5°C or lower. Twenty-five grams (114 mmol) of 2-bromobenzoyl chloride was added dropwise thereto while regulating the reaction temperature so as not to exceed 5°C. Thereafter, the reaction mixture was stirred at room temperature overnight. The resultant liquid reaction mixture was concentrated at ordinary pressure to distill off the dichloromethane or the excess diisopropylamine. Thereafter, toluene and water were added to the residue to conduct extraction. The organic layer was washed with saturated aqueous sodium chloride solution, subsequently dried with anhydrous magnesium sulfate, and then concentrated to isolate 2-bromo-N,N'-diisopropylbenzamide as colorless crystals. This reaction product was used as it was in the succeeding reaction without being purified. Subsequently, 7.5 g (26.3 mmol) of the 2-bromo-N,N'-diisopropylbenzamide obtained, 5.1 g (29.1 mmol) of 1-naphthylboronic acid, 150 mg (0.13 mmol) of tetrakis (triphenylphosphine)palladium, 38.5 g of 20% by weight aqueous sodium carbonate solution, and 60 mL of tetrahydrofuran were introduced into a 200-mL eggplant type flask. This reaction mixture was heated and stirred overnight with refluxing. The resultant mixture was cooled to room temperature. Thereafter, water was added thereto conduct extraction. The organic layer obtained was washed with saturated aqueous sodium chloride solution, subsequently dried with anhydrous magnesium sulfate, and then concentrated to obtain light-brown crystals. Furthermore, the crystals were purified by silica gel chromatography (solvent: hexane/toluene) to isolate 7.1 g of colorless crystals (yield, 82%). These crystals were ascertained to be the target compound through 1H-NMR spectroscopy.
References:
EP2006278,2008,A2 Location in patent:Page/Page column 26; 27
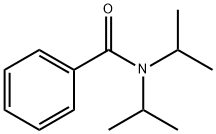
20383-28-2
67 suppliers
$60.00/500mg

79839-66-0
19 suppliers
$45.00/100mg

108-18-9
364 suppliers
$10.00/1g
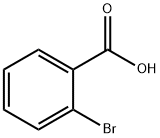
88-65-3
341 suppliers
$5.00/5g

79839-66-0
19 suppliers
$45.00/100mg
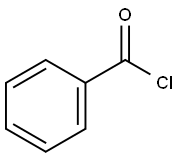
98-88-4
580 suppliers
$10.00/5g

79839-66-0
19 suppliers
$45.00/100mg

108-18-9
364 suppliers
$10.00/1g

79839-66-0
19 suppliers
$45.00/100mg