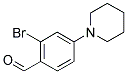
2-BROMO-4-PIPERIDIN-1-YL-BENZALDEHYDE synthesis
- Product Name:2-BROMO-4-PIPERIDIN-1-YL-BENZALDEHYDE
- CAS Number:886502-22-3
- Molecular formula:C12H14BrNO
- Molecular Weight:268.15
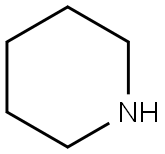
110-89-4
3 suppliers
$15.96/100ML
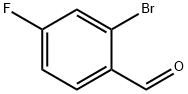
59142-68-6
277 suppliers
$14.00/25g

886502-22-3
8 suppliers
inquiry
Yield:886502-22-3 96%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 110; for 18 h;Inert atmosphere;
Steps:
2-Bromo-4-(piperidin- 1-yl)benzaldehyde (C2).
2-Bromo-4-fluorobenzaldehyde (2.0 g, 0.0099 mol), piperidine (1.03 mL, 0.0104 mol, 1.05 eq), potassium carbonate (1.57 g,0.0 114 mol, 1.15 eq) and anhydrous DMF (20 mL) were combined and the stirred reactionmixture was heated at 110 °C under nitrogen for 18 h. The reaction mixture was allowed tocool at room temperature, concentrated, and partitioned between water and EtOAc. Theorganic phase was separated, washed with brine, dried (Mg504), filtered, and concentrated.The crude product was purified by flash chromatography over 5i02 (40 g) with ahexane:EtOAc gradient (100:0 to 60:40) to give 2.55 g (96%) of the title compound as ayellow oil. ‘H NMR (300 MHz, CDC13): ? 10.07 (s, 1H), 7.78 (d, J = 8.9 Hz, 1H), 6.96 (d, J= 2.0 Hz, 1H), 6.80 (d, J = 8.8 Hz, 1H), 3.40 (s, 4H), 1.68 (s, 6H).
References:
WO2015/195486,2015,A1 Location in patent:Paragraph 0130