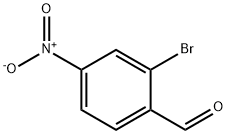
2-Bromo-4-nitrobenzaldehyde synthesis
- Product Name:2-Bromo-4-nitrobenzaldehyde
- CAS Number:5274-71-5
- Molecular formula:C7H4BrNO3
- Molecular Weight:230.02
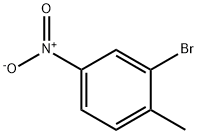
7745-93-9
289 suppliers
$5.00/5g

5274-71-5
73 suppliers
$35.00/250mg
Yield: 9%
Reaction Conditions:
Stage #1:2-bromo-4-nitrotoluene with chromium(VI) oxide;sulfuric acid;acetic anhydride in acetic acid at 5 - 10; for 1.5 h;Cooling with ice;
Stage #2: with sulfuric acid in ethanol;water for 1 h;Reflux;
Steps:
12.A Part A. 2-Bromo-4-nitrobenzaldehyde
2-Bromo-1-methyl-4-nitrobenzene (15.0 g, 69.4 mmol) was dissolved inacetic acid (112 mL) and acetic anhydride (105 mL, 1,113 mmol). The mixture was placed in an ice-water bath and concentrated sulfuric acid (15 mL, 281 mmol) was added slowly. Chromium(VI) oxide (34.7 g, 347 mmol) was then added in portionswhile maintaining the temperature of the reaction mixture between 5-10 ° C. The reaction mixture was stirred in an ice-bath for 1.5 h. The internal temperaturehovered between 5-10 °C. It was necessary to monitor the reaction temperature due to a delayed exotherm. At one point it was necessary to place the reaction mixture in an ice-acetone bath for a period of time to keep the temperature from exceeding 10°C. The contents of the flask were then poured into a 2 liter Erlenmeyer flask containing some ice. Cold water was then added to bring the total volume to 1500mL. The solid was collected on a Buchner funnel. The solid was washed with cold water until it was nearly colorless. The solid was suspended in cold 2% aqueous Na2CO3 solution (100 mL) and was thoroughly stirred. The solid was collected on a filter and was washed with cold water and partially dried on the Buchner funnel to give 8.3 grams of the diacetate intermediate.A mixture of 8.3 g of crude diacetate intermediate, ethanol (16 mL), water (16 mL), and conc. H2S04 (2.4mL) was heated at reflux for 1 h. The mixture was cooled to room temperature and the mixture was transferred to a separatory funnel containing saturated aqueous K2C03 (50 mL) solution. A fluffy solid formed. It was subsequently determined that this solid was not the product. The aqueous layer wasextracted with ethyl acetate (4 x 50 mL). The combined organic layers were washed with brine (25 mL), dried over MgSO4, filtered, and concentrated. The solid was purified by colunm chromatography on silica gel (5% -* 15% ethyl acetate in hexanes) to afford 2-bromo-4-nitrobenzaldehyde (1 .41 g, 9% yield) as a colorless fluffy solid: ‘H NMR (400 MHz, CDC13) ? 10.41 (s, 1 H), 8.51 (d, J=2.0 Hz, 1 H),8.25 (dd, J=8.4, 2.1 Hz, 1 H), 8.06 (d, J8.6 Hz, 1 H).
References:
BRISTOL-MYERS SQUIBB COMPANY;HARTZ, Richard A.;AHUJA, Vijay T.;BRONSON, Joanne J.;DZIERBA, Carolyn Diane;MACOR, John E.;NARA, Susheel Jethanand;RAJAMANI, Ramkumar WO2015/6100, 2015, A1 Location in patent:Page/Page column 75; 76
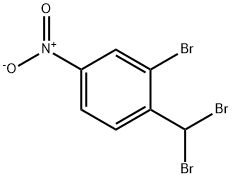
1313762-48-9
2 suppliers
inquiry

5274-71-5
73 suppliers
$35.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

5274-71-5
73 suppliers
$35.00/250mg
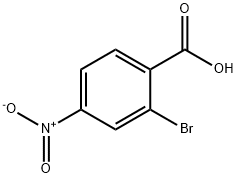
16426-64-5
153 suppliers
$5.00/250mg

5274-71-5
73 suppliers
$35.00/250mg
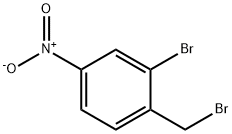
940-05-6
66 suppliers
inquiry

5274-71-5
73 suppliers
$35.00/250mg