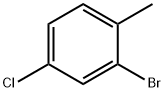
2-Bromo-4-chlorotoluene synthesis
- Product Name:2-Bromo-4-chlorotoluene
- CAS Number:27139-97-5
- Molecular formula:C7H6BrCl
- Molecular Weight:205.48
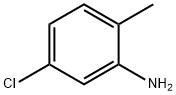
95-79-4
254 suppliers
$13.00/5g

27139-97-5
237 suppliers
$6.00/5g
Yield:27139-97-5 72%
Reaction Conditions:
Stage #1:5-chloro-2-methyl-benzenamine with hydrogen bromide in water for 0.333333 h;
Stage #2: with sodium nitrite in water at -5; for 1.5 h;
Stage #3: with hydrogen bromide;copper(I) bromide in water at 0 - 70; for 0.5 h;
Steps:
2
In a 3000 ml beaker 142 g (1.00 mol) of melted 2-methyl-4- chloroaniline were slowly added to 1200 ml of 23% aqueous HBr. This mixture was stirred for 20 min using a mechanical stirrer, cooled to -50C; and then a solution of 70.0 g (1.00 mol) Of NaNO2 in 400 ml of water was added dropwise for 1.5 h at this temperature. The diazonium reagent obtained was added in several portions to a solution of 144 g (1.00 mol) of CuBr in 400 ml of 47% HBr at O0C. The resulting mixture was warmed to 7O0C, stirred for 30 min at this temperature, and, then, cooled to room temperature. The product was extracted with 3 x 500 ml of methyl-ter/-butyl ether; and the combined extract was dried over K2CO3 and evaporated to dryness. Fractional distillation gave colorless oil, b.p. 81-84°C/7 mm Hg. Yield 148 g (72%).Anal. calc. for C7H6BrCl: C, 40.92; H, 2.94. Found: C, 41.00; H, 2.99.1H NMR (CDCl3): δ 7.45 (d, J= 1.8 Hz, I H, 2-H), 7.34 (dd, J= 6.0 Hz, J= 1.8 Hz, IH, 4-H), 7.12 (d, J= 6.0 Hz, I H, 5-H), 2.43 (s, 3H, Me).
References:
EXXONMOBIL CHEMICAL PATENTS INC. WO2007/70041, 2007, A1 Location in patent:Page/Page column 90
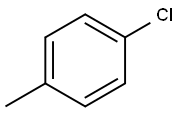
106-43-4
341 suppliers
$14.00/5g

27139-97-5
237 suppliers
$6.00/5g
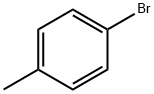
106-38-7
402 suppliers
$14.00/5g

27139-97-5
237 suppliers
$6.00/5g
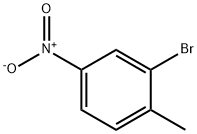
7745-93-9
293 suppliers
$5.00/5g

27139-97-5
237 suppliers
$6.00/5g
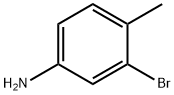
7745-91-7
321 suppliers
$6.00/5g

27139-97-5
237 suppliers
$6.00/5g